* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Multicellular trichomes in Arabidopsis - Development
Survey
Document related concepts
Tissue engineering wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell nucleus wikipedia , lookup
Cell encapsulation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Programmed cell death wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cytokinesis wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell growth wikipedia , lookup
Transcript
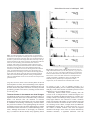
3931 Development 127, 3931-3940 (2000) Printed in Great Britain © The Company of Biologists Limited 2000 DEV0305 SIAMESE, a gene controlling the endoreduplication cell cycle in Arabidopsis thaliana trichomes Jason D. Walker1, David G. Oppenheimer2, Joshua Concienne1 and John C. Larkin1,* 1Department 2Department *Author of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803, USA of Biological Sciences and Coalition for Biomolecular Products, University of Alabama, Tuscaloosa, AL 35487, USA for correspondence (e-mail: [email protected]) Accepted 3 July; published on WWW 22 August 2000 SUMMARY Cell differentiation is generally tightly coordinated with the cell cycle, typically resulting in a nondividing cell with a unique differentiated morphology. The unicellular trichomes of Arabidopsis are a well-established model for the study of plant cell differentiation. Here, we describe a new genetic locus, SIAMESE (SIM), required for coordinating cell division and cell differentiation during the development of Arabidopsis trichomes (epidermal hairs). A recessive mutation in the sim locus on chromosome 5 results in clusters of adjacent trichomes that appeared to be morphologically identical ‘twins’. Upon closer inspection, the sim mutant was found to produce multicellular trichomes in contrast to the unicellular trichomes produced by wild-type (WT) plants. Mutant trichomes consisting of up to 15 cells have been observed. Scanning electron microscopy of developing sim trichomes suggests that the cell divisions occur very early in the development of mutant trichomes. WT trichome nuclei continue to replicate their DNA after mitosis and cytokinesis have ceased, and as a consequence have a DNA content much greater than 2C. INTRODUCTION Even a casual inspection reveals that patterns of cell division in plants are highly organized. Division is concentrated in meristematic regions exhibiting distinctly nonrandom division patterns. For example, shoot meristems are organized into recognizable cell layers maintained by the orientation of cell division planes (Steeves and Sussex, 1989). Cell differentiation typically occurs following cessation of the mitotic cell cycle, and the final morphology is generated by polarized cell expansion. These observations suggest that cell division patterns are tightly coupled to the generation of form. In contrast, a number of observations have shown that plant morphogenesis is surprisingly independent of cell division patterns (Kaplan and Hagemann, 1991). Embryo development and organogenesis can proceed in the absence of fixed cell lineages (Poethig and Sussex, 1985; McDaniel and Poethig, 1988; Irish and Sussex, 1992). In a now classic series of studies, the initial development of leaf primordia and lateral This phenomenon is known as endoreduplication. Individual nuclei of sim trichomes have a reduced level of endoreduplication relative to WT trichome nuclei. Endoreduplication is also reduced in dark-grown sim hypocotyls relative to WT, but not in light-grown hypocotyls. Double mutants of sim with either of two other mutants affecting endoreduplication, triptychon (try) and glabra3 (gl3) are consistent with a function for SIM in endoreduplication. SIM may function as a repressor of mitosis in the endoreduplication cell cycle. Additionally, the relatively normal morphology of multicellular sim trichomes indicates that trichome morphogenesis can occur relatively normally even when the trichome precursor cell continues to divide. The sim mutant phenotype also has implications for the evolution of multicellular trichomes. Key words: Trichome, Arabidopsis, Endoreduplication, Endoduplication, Cell cycle, Morphogenesis, Differentiation, Plant development, Leaf root primordia was shown to proceed even when mitosis was blocked (Haber, 1962; Foard et al., 1965; Foard, 1971). In more-recent genetic studies, mutants in maize (Smith et al., 1996; Reynolds et al., 1998) and Arabidopsis (Torres-Ruiz and Jürgens, 1994; Traas et al., 1995) have been identified that dramatically alter the orientation of cell divisions without causing major changes in the shape of the resulting tissue. In addition, experimental manipulation of regulatory components of the cell cycle has revealed that plants have a remarkable ability to compensate for altered cell division rates. Hemerly and co-workers (Hemerly et al., 1995) introduced a dominantnegative mutant of a mitotic cyclin-dependent kinase gene into transgenic tobacco plants, which resulted in plants with essentially normal morphology, but fewer and larger cells. In another study, overexpression of a mitotic B-cyclin in Arabidopsis resulted in an increase in the number of cells in the roots (Doerner et al., 1996). Although the roots were longer, they were otherwise morphologically normal. These observations suggest that cell division patterns may not play a direct causal role in morphogenesis. 3932 J. D. Walker and others The majority of these studies have been at the level of whole organ or tissue development, and much less is known about coordination of morphogenesis and cell division at the singlecell level. One of the most thoroughly studied plant cell differentiation pathways is the development of trichomes (leaf hairs) in Arabidopsis thaliana (Marks, 1997; Hülskamp et al., 1999). Arabidopsis trichomes are single cells that extend from the epidermis of leaves and stems. On leaves, these cells have an unusual branched shape resulting from a dramatic program of cellular morphogenesis. On stems, trichomes are generally unbranched. The single nucleus of a wild-type (WT) trichome continues to replicate its genomic DNA during differentiation, reaching nuclear DNA levels of 20C to 32C (Melaragno et al., 1993; Hülskamp et al., 1994), a process known as endoreduplication. Many genes involved in trichome development have been identified and several of these affect endoreduplication. Mutations at the glabra3 (gl3) locus reduce endoreduplication, trichome branching and trichome cell size (Hülskamp et al., 1994). In contrast, mutations at the triptychon (try) and kaktus (kak) loci increase endoreduplication, trichome branching and trichome cell size (Hülskamp et al., 1994). Trichomes of try mutants also occur in clusters in the epidermis, suggesting that the TRY gene plays a role in determining trichome cell fate in addition to its role in trichome differentiation. The GLABRA1 (GL1) gene is another cell fate determination gene that may also play a role in the control of endoreduplication, although published evidence is contradictory (Schnittger et al., 1998; Szymanski and Marks, 1998). Endoreduplication is an alternate version of the cell cycle that occurs in a wide variety of organisms (Nagl, 1976; Barlow, 1978; Traas et al., 1998). During endoreduplication cycles (endo cycles), nuclear DNA is replicated without mitosis or cytokinesis, resulting in cells with a DNA content much greater than 2C. In angiosperms, endoreduplication is particularly common and occurs in a wide variety of tissues and cell types (Barlow, 1978; Galbraith et al., 1991; Gendreau et al., 1997), including agriculturally important tissues such as maize endosperm (Kowles and Phillips, 1985). Animal cell types that undergo endoreduplication include many Drosophila larval tissues (Orr-Weaver, 1994), some molluscan neurons (Chase and Tolloczko, 1987), mammalian megakaryocytes (Angchaisuksiri et al., 1994) and placental trophoblast cells (Sarto et al., 1982). The function of endoreduplication is unknown, although proposed roles include gene amplification, radiation resistance and cell differentiation (Nagl, 1976; Barlow, 1978). In plants, there is often a correlation between the final volume of a differentiated cell and its DNA content (Barlow, 1978; Melaragno et al., 1993), but evidence of a functional link between DNA content and cell volume is lacking. The control of endoreduplication, and its relationship to cell differentiation and morphogenesis, is unclear, and until recently, has been little studied. In this study, we describe a mutation in a newly identified gene, SIAMESE (SIM), that results in multicellular trichomes, in contrast to the unicellular trichomes of WT plants. Nuclei in the multicellular trichomes have reduced levels of endoreduplication. Double mutants between sim and either gl3 or try are consistent with a role for sim in endoreduplication. SIM appears to encode a repressor of mitosis required for normal trichome endo cycles. MATERIALS AND METHODS Plant growth and mutant isolation All plants were grown as described previously (Larkin et al., 1999), except that for studies of hypocotyl endoreduplication, surfacesterilized seeds were sown on agar containing Murashige and Skoog salts, and the plates were incubated vertically for eight days, either in continuous light or in darkness. Dark-grown conditions were obtained by wrapping the plates in aluminum foil and placing them in a drawer. The sim mutant was identified in a screen of ethyl-methanesulfonate (EMS) mutagenized wild-type Columbia M2 seeds purchased from Lehle Seeds (Roundrock, TX). The initial mutant was crossed to Columbia (Col-1) wild-type once, backcrossed once and sim mutant plants were selected from the self-progeny of the backcrossed plants. No phenotypes other than the sim phenotype were observed segregating in any families derived from the original sim mutant. These plants were used for the initial studies. An additional backcross has now been performed, and the sim phenotype in the self-progeny of this backcross is indistinguishable from the phenotype of first backcross sim plants. Other Arabidopsis strains used in this study were gl3-1 Landsberg erecta (Ler) background, Koornneef et al., 1982), a gl3-1 line that had been crossed three times to Col before selfing (J. L. and J. W., unpublished), try-JC (Col background, Larkin et al., 1999) and a 35SGUS::Ac line (Lawson et al., 1994; Larkin et al., 1996). Complementation and genetic mapping For the complementation test, sim was crossed to try-JC plants, and the resulting F1 plants were examined. sim fully complemented the recessive trichome clustering phenotype of try. The increased trichome branching phenotype of try is incompletely dominant (J. C. L., unpublished). sim/+ try/+ heterozygotes exhibited a slight increase in branching, indistinguishable from that observed in try/+ heterozygotes. In the F2 derived from the double heterozygotes, 39 WT, 12 sim, 12 try, and 8 sim try plants were observed (χ2=1.9, P>0.5 for 9:3:3:1 segregation ratio), demonstrating that sim and try were unlinked. Plants for mapping experiments were produced from crosses between homozygous sim plants and WT plants of the either the RLD or Ler ecotypes. Total nucleic acids were prepared from F2 plants that had the sim phenotype, and PCR amplification with primer-pairs for various molecular markers was performed essentially by standard methods (Bell and Ecker, 1994). Molecular markers tested for linkage were nga63, nga128, nga168, nga6, nga8, nga172, nga1139, AG, DET1, AtS0191, nga249, and nga158 (Konieczny and Ausubel, 1993; Bell and Ecker, 1994). Significant linkage was found between sim and nga158 (8 of 128 chromosomes recombinant, χ2=98.0, P<0.005). Map order was determined by the pattern of recombination with the linked marker nga249. None of the other markers showed significant linkage. sim has been further mapped to two BAC clones distal to nga158 that total approximately 200kb. We currently have 20 sim recombinants mapping within this region, and all of them retain all aspects of the sim phenotype. It is thus likely that the sim phenotype is due to a mutation in a single locus. Phenotypic analysis Cryo-scanning electron microscopy (see Figs 1C, 2, 5) was performed as described by Luo and Oppenheimer (1999). The scanning electron microscopy in Fig. 1A,B was performed as previously described (Larkin et al., 1999). All images were electronically processed in Adobe Photoshop, adjusting only for brightness and contrast. Samples for anatomical sectioning were fixed, embedded, sectioned and stained essentially as described by Fernandez and co-workers (Fernandez et al., 2000), using standard methods (Ruzin, 1999). For 4′,6-diamidino-2-phenylindole (DAPI) staining, leaves were fixed in FAA (50% ethanol, 5% glacial acetic acid, 10% formalin) and stained for 15 minutes to 2 hours with 20 µg/ml DAPI in McIlvaine’s Multicellular trichomes in Arabidopsis 3933 buffer (60 mM citric acid, 80 mM Na2HPO4, pH 4.1), then washed three times for 15 minutes each with McIlvaine’s buffer. Samples were mounted in 50% glycerol in McIlvaine’s buffer and observed via epifluorescence on a Nikon Microphot FXA microscope. Fluorescence of individual nuclei was measured with a Nikon spot photometer attachment. Background readings from an area in the specimen without a nucleus were subtracted from the fluorescence of each nucleus prior to further analysis. In some experiments, fluorescence from guard cell nuclei in epidermal peels mounted on the same slide were obtained. For deconvolution microscopy, samples stained with DAPI and mounted as described above were observed with a 63× oil immersion objective on a Leica DMRXA Research Microscope. Data were collected and analyzed using the Slidebook software from Intelligent Imaging Innovations (Denver, CO). Nuclear volumes were estimated from nuclear diameters assuming that the nucleus was roughly spherical, which appears to be true for trichome and epidermal cell nuclei in the early developmental stages examined here. Guard cell nuclei have been reported to have a 2C DNA content (Melaragno et al., 1993), and have often been used to standardize trichome DNA values. However, we have obtained variable results when normalizing to guard cell relative fluorescence units. In our hands, guard cell nuclei have a fluorescence level only slightly above background, which results in a small number that includes a relatively large amount of variation. Much more consistent results were obtained when samples were normalized to the mean fluorescence intensity of a sample of Col trichome nuclei included in the same experiment. For the trichome experiments reported here, measured fluorescence levels for individual nuclei were divided by the mean value for Col trichome nuclei, and the resulting number was multiplied by 32. Although our data are expressed as relative fluorescence units (RFU) normalized to Col, we have artificially set Col at 32 RFU. Our RFU values should thus be roughly comparable with the C values reported by others. A Col trichome nuclear DNA content of 32C is consistent with the results we have obtained in experiments where guard cell DNA content was measured. Data for epidermal pavement cells and hypocotyl nuclei were normalized similarly, using mean C values of pavement cells from Fig. 2A of Melaragno et al. (1993) and of lightgrown hypocotyls from Fig. 3A of Gendreau et al. (1998). Because the DNA content of endoreplicated nuclei is unlikely to follow a normal distribution, pairwise nonparametric Mann-Whitney U-tests were performed for the genetically relevant comparisons using the Statistica software package (StatSoft, Inc., Tulsa, OK). The standard Bonferroni technique was used to obtain P values corrected for the multiple comparisons conducted (Rice, 1989). The results of one set of experiments are reported here; all results on trichome nuclear DNA content have been replicated in at least one independent experiment. Analysis of L1 clonal sectors For the production of clonal sectors, a sim 35SGUS::Ac strain was constructed by crossing sim plants with a previously described 35SGUS::Ac transgenic line (Lawson et al., 1994). Leaf tissue was histochemically stained for β-glucuronidase (GUS) activity as described previously (Larkin et al., 1996), and sectors on first leaves were examined with DIC optics at 200× magnification. Sectors were chosen for analysis if they extended at least 50% of the length of the leaf. This type of sector results from transposon excision prior to the initiation of trichome development (Larkin et al., 1996). Trichome clusters were included in the analysis if at least one trichome in the cluster was stained, and at least one trichome in the cluster was immediately adjacent to unstained cells (i.e., was at the edge of the stained sector). To estimate the probability that a clonal sector boundary would be expected to include only one trichome in a cluster of two trichomes, we took advantage of the observation that at the time of trichome initiation, cells in the epidermis approximate a hexagonally packed array, i.e., each cell is surrounded by an average of six neighbors (Larkin et al., 1996). In this case, a pair of adjacent trichomes (the cluster) would be surrounded by eight other cells. It is straightforward to calculate that a total of 262 combinations occur (Boas, 1966) in which both trichomes are labeled, and one, two, three, four, five, six or seven of the surrounding cells are labeled. These combinations correspond to the number of ways that a sector boundary can pass adjacent to a cluster of two trichomes and include both trichomes within the GUS-expressing sector. It is more difficult to calculate the number of combinations in which only one of the trichomes is included in the sector, owing to the complication that the stained cells must be contiguous. However, direct counting revealed a minimum of at least 224 ways that a sector could include only one trichome of the cluster. Thus, there are a total of at least 486 ways that a developing trichome cluster could contact the edge of a clonal sector. Thus we would expect that in a minimum of 224/486, or 46%, of trichome clusters at a sector border, the sector boundary would pass between the two trichomes in the absence of any constraint on the clonal relationship of the two trichomes. Construction of double mutants Double mutants were produced by crossing the individual mutant homozygotes and examining trichome phenotypes in the F2. Suspected double mutants were crossed individually to each parental mutant and confirmed by the absence of complementation in the resulting progeny. The double mutants were also allowed to self, and the self-progeny scored to show that the double mutant phenotype breeds true. RESULTS sim mutants produce multicellular trichomes On WT leaves, virtually all trichomes occur singly (Fig. 1A). Instances where two or more adjacent trichomes occur with no intervening cells have been termed trichome clusters (Larkin et al., 1994). A mutant producing frequent clusters of trichomes was identified in a screen of EMS-mutagenized plants (Fig. 1B). Because many of the mutant trichome clusters looked like ‘twins’ with trichomes of nearly identical morphology joined at the base, the gene identified by this mutation was named SIAMESE (SIM). Approximately 65% of the trichomes on sim first leaves were adjacent to another trichome (Table 1); however, the number of sites on the leaf Table 1. Number of trichomes, trichome initiation sites (TIS) and percentage of trichomes in clusters Genotype Trichomes per leaf (mean±s.d.) TIS per leaf (mean±s.d.) % in clusters Col sim gl3* try sim gl3‡ sim try 28.4±4.8 47.3±9.8 23.3±5.3 30.7±5.2 16.9±5.31 n.c.§ 28.3±4.7 31.9±6.06 21.9±5.1 27.4±4.8 3.5±5.2 26.2±2.3 0.7 5.3 10.7 27.4 41.4 >99 Data are based on counts of all of the trichomes on the adaxial surface of ten first leaves per genotype. Trichomes were considered to be in a cluster if they were immediately adjacent to another trichome with no intervening cells. *gl3-1 allele crossed three times to Col before selfing. ‡gl3-1 allele in Ler background was used in construction of the double mutant. §n.c., not counted. The trichomes associated with each sim try cluster were too crowded to count (Fig. 5D). Most TIS of this genotype appeared to contain four to ten trichomes that were in contact with other epidermal cell. One instance of a solitary trichome was observed in this sample. 3934 J. D. Walker and others Fig. 2. Cross sections through sim multicellular trichomes. (A) Multicellular trichome with relatively normal morphology. (B) Multicellular trichome with altered morphology and several division planes. Arrow indicates a cell junction similar to those indicated in Fig. 1B. Bar, 75 µm. Fig. 1. siamese (sim) trichomes are multicellular. (A) A WT unicellular trichome. (B) A cluster of two sim trichomes, each of which consists of two cells. Cell junctions are indicated by white arrows. (C) Close-up of basal junction of two sim trichomes. (D) DAPI-stained multicellular stem trichome with six nuclei, separated by cell walls. Bars, 100 µm (A); 100 µm (B); 15 µm (C). containing at least one trichome (trichome initiation sites, TIS) was approximately the same as was seen on WT leaves. No other aspects of plant growth and development appeared to be affected. In particular, fertility, leaf expansion, and development of other epidermal cell types, root hairs and root cortical cells all appeared to be unaffected by the mutation. The sim phenotype superficially resembled the trichome clustering phenotype of try mutants. However, unlike try, the individual trichomes in each cluster were typically joined well above the epidermal surface (Fig. 1C). Additionally, try trichomes had an increased number of trichome branches, whereas sim trichomes had a WT branching pattern. Complementation tests and segregation analysis demonstrated that sim was not allelic to try (see Materials and Methods). The sim gene was mapped to a position approximately 6 cM distal to nga158 on chromosome 5. Closer inspection revealed that many of the sim trichomes were multicellular (Fig. 1B,D; Fig. 2A,B – arrows in Fig. 1B indicate cell junctions). Fig. 2A shows a cross section of a multicellular trichome of relatively normal morphology similar to the trichomes in the cluster shown in Fig. 1B, while Fig. 2B shows a cross section of a trichome with distorted morphology and relatively disorganized planes of division. On stems, as many as 15 cells per trichome have been observed, although most leaf trichomes are composed of only two or three cells. Staining of trichomes with the fluorescent DNA stain DAPI demonstrated that each of the cells in a multicellular trichome has its own nucleus, and each cell in a multicellular sim trichome has only a single nucleus (Fig. 1D). Development of sim trichomes Cell division has never been observed in hundreds of developing WT trichomes that have been examined (J. C. L. and D. G. O., unpublished). In contrast, cell division was often observed early in the development of sim trichomes (Fig. 3A). Divisions occurring shortly after initiation of trichome development appeared to result in two fully formed adjacent trichomes, producing the trichome clusters that were initially observed (Figs 1B,C, 3B). Divisions occurring later resulted either in cells that reiterated the trichome branching pattern and other aspects of the morphogenic program while attached to another trichome cell (Fig. 3C), or in multicellular trichomes with relatively normal morphology (Figs 1B, 3D). These divisions did not appear to affect later stages of trichome differentiation such as branching and secondary cell wall thickening, although some cell boundaries within a mutant trichome did appear to be correlated with branch junctions Fig. 3. Development of sim trichomes. (A) Early stage of development, showing evidence of recent cell divisions in a developing trichome. (B) Slightly later stage in the development of a sim trichome cluster. (C) Same stage as B, single trichome composed of two cells. Upper cell is reiterating trichome branching pattern. (D) Various stages of sim trichome development, including later stages. Arrow indicates cell junction. Bars, 10 µm (A); 15 µm (B); 15 µm (C); 50 µm (D). Multicellular trichomes in Arabidopsis 3935 Fig. 4. sim trichome clusters are clonal in origin. A GUS-stained clonal sector in the epidermis resulting from Ac transposon excision is outlined on the surface of the leaf. This sector ran from the base of the leaf to a point on the leaf margin about 60% of the way to the tip. All four trichomes in the cluster are stained. The dotted line indicates where the sector boundary passes behind the four clustered trichomes. Guard cells are particularly useful in following sector boundaries, because guard cells and trichomes stain more intensely than other cell types. White arrows show unstained guard cells outside the sector. Black arrows show stained guard cells within the sector. An independent stained vascular sector passes below the epidermal sector, and staining is also visible in the mesophyll in the upper right. Note that the guard cells above the stained mesophyll cells are not stained, indicating that this staining is subepidermal. These two subepidermal sectors are not relevant to the analysis of the epidermal sector. (Fig. 1B). On leaves, there was no obvious pattern to the cell division planes. On stems, where no branching occurs, adjacent trichomes were observed, presumably resulting from early cell divisions, but the later planes of division were always oriented periclinally (parallel to the surface), resulting in linear chains of cells (Fig. 1D). Trichome clusters in sim mutants are clonal lineages Two different models can be invoked to explain the clusters of adjacent trichomes observed on sim mutants. If they result from mitotic cell divisions after the initiation of trichome development, then all of the trichome cells in a cluster should be immediate clonal siblings. Alternatively, trichome clusters may result from a failure of cell signaling during the selection of trichome precursor cells. The latter model appears to explain the trichome clusters observed in try mutants (Schnittger et al., 1999). Although observations of developing sim trichomes (Fig. 3) would favor the first model, many regulatory factors Fig. 5. Relative DNA content of DAPI-stained trichome nuclei of single and double mutants. (A) Col (WT), (B) sim, (C) gl3, (D) sim gl3, (E) try, (F) sim try. The degree of fluorescence is expressed in terms of relative fluorescence units (RFU) normalized to the mean fluorescence of Col. These RFU values have been adjusted to be roughly comparable to published C values (see Materials and Methods), but they should only be interpreted as relative comparisons, not as measurements of absolute DNA contents. are known to play a role in multiple processes in a developmental pathway, and we considered the possibility that SIM plays a role in the selection of trichome precursor cells in addition to its role in regulating mitosis. Genetically marked clonal sectors resulting from the excision of an Ac transposon from a 35SGUS transgene were used to test whether all adjacent trichomes in a cluster were clonally related, as described previously (Larkin et al., 1996). Thirty-eight instances were observed in which a trichome cluster was located at the border of a GUS-stained sector and at least one trichome was stained. If there were no constraints on cell lineage of the cluster, a simple model (see Materials and Methods) suggests that we would expect 46%, (17.5) of the clusters to include trichome cells from the adjacent unstained cell lineage. None of the 38 clusters included any unstained trichomes, a significant difference from the 3936 J. D. Walker and others Fig. 6. Nuclear size of WT and sim trichome nuclei during early trichome development in the leaf protoderm. Arrows indicate trichome nuclei; arrowheads show undifferentiated protodermal nuclei. (A) sim protoderm containing a cluster of three developing trichomes. The trichome closest to the bottom edge has begun to expand out from the plane of the epidermis (stage 2 of Szymanski et al., 1998), while the remaining two trichomes in the cluster are dome-shaped (stage 1 of Szymanski et al., 1998). (B) WT protoderm containing a stage 1 trichome. In both (A) and (B), the trichome nuclei are larger than surrounding undifferentiated protodermal cells. Bar, 10 µm. expectation for the null hypothesis of no clonal constraint (χ2=32.4, P<0.001). It is important to recognize that the model of clonal boundaries used to generate the expected value used here is an idealization. For example, real sectors are not oriented at random on the leaf, and this could bias the frequency with which all of the trichomes in a cluster would be included within the sector. However, we have noted that the individual trichomes in sim clusters appear to be randomly oriented with respect to the leaf axis (J. W. and J. L., unpublished observations), which would tend to counteract bias of this type. Also, some of the observed trichome clusters included more than two trichomes, which would tend to increase the chance that one or more trichomes would not be included in the sector. A particularly striking example of a cluster of four trichomes at a sector boundary, all stained, is shown in Fig. 4. In this case, and in several others, the sector boundary appears to make a substantial detour to include all of the trichomes in the cluster. Taken together, these results indicate that sim trichome clusters arise through divisions of a trichome precursor cell, and provide no evidence in favor of a role for sim in signaling pathways involved in the selection of precursor cells. sim mutants have altered endoreduplication WT trichomes have been reported to have nuclear DNA levels of approximately 20C to 32C (Melaragno et al., 1993; Hülskamp et al., 1994). In three independent experiments, we found that sim nuclei had significantly less DNA than WT (P<0.001). The results of one such experiment are shown in Fig. 5. These data indicate that sim trichome nuclei have about one-third the DNA content of WT trichome nuclei (Fig. 5A,B). Nuclei of multicellular trichomes had slightly less DNA than nuclei of unicellular sim trichomes (Table 2). Within the multicellular trichomes, there did not appear to be a difference in nuclear DNA content along the proximo-distal axis of the trichome (Table 2). Nuclei of the cells attached directly to the epidermis (basal cells) did not have a significantly different DNA content than nuclei of other cells within the multicellular trichomes (P=0.755). Fig. 7. Relative DNA content of DAPI-stained hypocotyl nuclei of WT and sim grown in the light or dark. (A) A WT DAPI-stained dark-grown hypocotyl. (B) A sim DAPI-stained dark-grown hypocotyl. (C-F) represent the pooled measurements of nuclei of the hypocotyl epidermis and cortex of the following genotypes and treatments: (C) WT grown in light, (D) sim grown in light, (E) WT grown in dark and (F) sim grown in dark. RFU, relative fluorescence units. In trichomes of morphological stage 1 and early stage 2, as described by Szymanski et al. (1998: stage 1, radial expansion within epidermis; stage 2, expansion from epidermis prior to branching), both sim (Fig. 6A) and WT (Fig. 6B) developing trichomes had nuclear volumes of approximately 100-150 µm3, in contrast to ordinary protodermal cells showing no signs of trichome development (Fig. 6A,B), which had nuclear volumes of approximately 12 to 36 µm3. Several of the sim developing trichomes observed were in clusters of adjacent trichomes (Fig. 6A), and their nuclei had similar nuclear volumes to isolated sim trichomes and WT trichomes. We did not observed a Multicellular trichomes in Arabidopsis 3937 Fig. 9. Model for role of SIM in endoreduplication. SIM is hypothesized to encode a repressor of mitosis that is necessary to establish the endo cycle. GL3 is hypothesized to function in the endo cycle by activating and inhibiting S phase, respectively. Pointed arrows indicate activation, and blunt arrows indicate inhibition. Fig. 8. Double mutant phenotypes. (A) gl3 trichome, (B) sim gl3 trichome cluster with a multicellular trichome (arrow), (C) try trichome cluster and (D) sim try trichome cluster. Bars, 100 µm (A); 70 µm (B); 100 µm (C); 100 µm (D). mutant trichome just after a division that generates a true multicellular trichome in this analysis (i.e., not just a cluster of trichomes, but rather multicellular in the aerial part of the trichome). Thus, although it is clear that sim trichomes can undergo some degree of endoreduplication early in trichome development, we do not yet understand the timing of the divisions generating the aerial multicellular class of trichomes relative to the timing of endoreduplication. We have also examined endoreduplication in other tissues. Epidermal pavement cell nuclei undergo endoreduplication, although to a lesser degree than trichome nuclei (Melaragno et al., 1993). In contrast to the situation for trichome nuclei, sim epidermal pavement cell nuclei did not have a decreased amount of DNA relative to WT (Table 2, P=0.21). Another tissue with a well-documented pattern of endoreduplication is the Arabidopsis hypocotyl (Gendreau et al., 1997). When grown in the dark, WT hypocotyl cells undergo two to three rounds of endoreduplication, while in the Table 2. Endoreduplication in multicellular and unicellular sim trichomes and epidermal pavement cells Cell type sim trichomes unicellular multicellular (all)* basal cell other cells WT trichomes (unicellular) sim pavement cells WT pavement cells Mean RFU±s.d. 5.5±11.11 11.1±8.1 11.3±8.9 11.0±7.4 32.0±15.8 3.9±9.6 3.4±2.0 Median RFU 2.2‡ 9.1‡ 8.2 9.9 30.1 2.81 3.1 light, they undergo approximately one less round of endoreduplication. A phytochrome-dependent pathway acts to repress one round of endoreduplication in the light (Gendreau et al., 1998). WT and sim hypocotyl epidermal and cortical cell nuclei had similar levels of DNA when grown in the light (Fig. 7C,D; Table 3). However, when grown in the dark, sim hypocotyl nuclei failed to undergo the additional endoreduplication seen in WT hypocotyl nulcei grown in the dark (Fig. 7A,B,E,F; Table 3). The sim mutant thus affects at least one endoreduplication pathway in addition to trichomes, but some endoreduplication pathways remain unaffected by the mutant. Genetic interactions of sim with two mutations that alter endoreduplication Double mutants were constructed between sim and two mutations that affect endoreduplication, gl3 and try. Trichomes on gl3 plants were smaller, had reduced branching, and reduced nuclear DNA content relative to WT (Figs 5C, 8A). Trichomes on gl3 plants also exhibited more clustering than WT trichomes (Table 1). Trichomes on sim gl3 plants resembled gl3 trichomes, except that some multicellular trichomes occurred (Fig. 8A,B, arrow indicates a cell junction). The frequency of trichome clusters on leaves of sim gl3 plants was 41.4%, a value intermediate between that of the gl3 and sim single mutants (Table 1). The degree of endoreduplication in sim gl3 trichome nuclei was significantly less than that of the gl3 single mutant (Fig. 5C,D; P<0.01), and was greater than that of the sim single mutant although not significantly (Fig. 5B,D; P=0.185). The primarily additive nature of the sim gl3 double n 33 80 38 42 42 68 102 n, number of nuclei; RFU, relative fluorescence units (normalized to the WT mean as described in Materials and Methods). The basal cell in a sim multicellular trichome are the cell that is directly attached to the epidermis. *In this analysis, trichomes were considered multicellular only if the aerial parts of the trichome were multicellular, i.e., trichomes in clusters joined at the base were included in the unicellular class. ‡These two samples differ significantly (P=0.045). Table 3. Hypocotyl nuclear DNA content and hypocotyl length in sim and WT plants grown in the light or in the dark Genotype WT sim Treatment Light Dark Light Dark Mean nuclear RFU ±s.d. (median) 5.6±2.9 (5.0)* 8.2±3.1 (8.1)*,‡ 5.9±3.3 (5.8) 4.9±3.1 (4.2)‡ n 56 49 92 56 Mean hypocotyl length (mm)±s.d. 2.2±0.4 22.1±2.8 2.4±0.3 24.4±2.2 Fluorescence of epidermal and cortical nuclei of hypocotyls was measured as described in Materials and Methods. RFU, relative fluorescence units, normalized to the WT mean, as described in Materials and Methods. *These two samples differ significantly (P<0.001). ‡These two samples differ significantly (P<0.001). 3938 J. D. Walker and others mutant phenotype suggests that these two genes reduce endoreduplication via different mechanisms. The phenotype of sim try trichomes was particularly striking. try single mutants produce some clusters of adjacent trichomes, apparently by affecting cell signaling during trichome initiation (Schnittger et al., 1999). In addition, try had effects on later aspects of trichome development, resulting in increased cell size, increased branching and increased nuclear DNA content (Figs 5E, 8C). The sim try double mutants produced large clusters of highly multicellular trichomes (Fig. 8D). Virtually all of the leaf trichomes on these plants were in clusters, a higher frequency than was observed in either individual mutant (Table 1). The sim try double mutants exhibited a relative DNA content similar to that found in the sim single mutant (Fig. 5B,F; P=0.56), and much less than the increased DNA content of try mutants (Fig. 5E,F; P<0.001). This indicates that sim is epistatic to try with regard to the degree of endoreduplication. These results are consistent with the hypothesis that sim and try function in the same pathway. DISCUSSION The presence of numerous multicellular trichomes on sim plants (Figs 1, 2, 3) demonstrates that trichome differentiation is not dependent upon the cessation of cell division. Furthermore, the relatively normal morphology of some multicellular sim trichomes indicates that cellular morphogenesis can occur surprisingly normally in the presence of continued cell division. In unbranched stem trichomes, division planes are periclinal once the trichome has begun to expand. Trichome clusters do occur on the stem, perhaps because anticlinal division planes were already established in some cells prior to commitment to the trichome pathway. In multicellular branched leaf trichomes, division planes are much more variable, perhaps because the branching mechanism interferes with the regular establishment of division planes. These results are consistent with the view that cell division is not essential for the generation of plant form, even at the cellular level (Kaplan and Hagemann, 1991). A simple model to explain the sim mutant phenotype is shown in Fig. 9. SIM is proposed to encode a repressor of mitosis that functions in the establishment of the endoreduplication cell cycle. In addition to the observation that sim trichomes continue to divide, several lines of evidence support this model. Trichome clusters in sim are clonal, consistent with the possibility that they are generated by division of a committed trichome precursor cell. The DNA content of sim trichome nuclei is reduced relative to WT, consistent with a role in the endo cycle. Although we have not measured C values of nuclei directly (see Materials and Methods), comparison of our data with the data of others suggests that most sim trichome nuclei are arrested with DNA contents of 4-8C. The sim mutation is also epistatic to try with regard to endoreduplication, consistent with the hypothesis that these two genes function in the same pathway. Although sim affects endoreduplication in trichomes, not all endoreduplication pathways are affected. Endoreduplication in epidermal pavement cells is unaltered, as is endoreduplication in light-grown hypocotyls. However, the additonal level of endoreduplication seen in dark-grown hypocotyls is absent in sim mutants. We do not yet know if sim is interfering with a specific phytochrome-repressed pathway in hyptocotyl development (Gendreau et al., 1998), or whether it is disrupting endoreduplication by a more general mechanism. This question is currently under investigation. The endoreduplication cell cycle has been most thoroughly studied in Drosophila melanogaster (Orr-Weaver, 1994). The first larval endo cycles begin after mitosis 16 during embryo development. Endoreduplication occurs in tissue-specific domains, and S phases alternate with a single G phase, suggesting that endo cycles are highly regulated (Smith and Orr-Weaver, 1991). Cyclin E appears to play an important role in promoting endo cycles (Knoblich et al., 1994; Sauer et al., 1995), while escargot, cdc2, Cyclin A and Myb appear to act as inhibitors of endoreduplication (Sauer et al., 1995; Hayashi, 1996; Katzen et al., 1998). In addition, fizzy-related functions as an inhibitor of mitotic cyclins during endo cycles, and has been proposed to be involved in the degradation of these cyclins (Sigrist and Lehner, 1997). Little was known about the control of endoreduplication in plants until recently (Traas et al., 1998). In maize endosperm, biochemical evidence has been obtained for two separable endoreduplication-promoting factors: an M phase inhibitor and an increase in S phase-related protein kinase activity (Grafi and Larkins, 1995). In addition, altered phosphorylation of a retinoblastoma homolog correlates with the onset of endoreduplication (Grafi et al., 1996). In animals, the phosphorylation state of retinoblastoma protein controls entry into S phase. The Arabidopsis thaliana cyclindependent kinase cdc2b is expressed in endoreplicating cells, and may play a role in this process (Segers et al., 1996). These studies suggest that the control of endoreduplication in plants is similar to the situation in animals, with different factors acting to stimulate S phase and inhibit M phase of the cell cycle. SIM is a good candidate for a gene encoding a component of this mitosis-inhibiting pathway during the endo cycle, similar to the role of fizzy-related in Drosophila (Sigrist and Lehner, 1997). GL3 encodes a basic-helix-loop-helix protein similar to the maize R gene, and probably functions in the initiation of trichome development (M. Brown and J. C. L., unpublished). GL3 is a good candidate to function as a transcription factor promoting S-phase during trichome development (Fig. 9). If GL3 were necessary for endoreduplication to occur, one might expect that GL3 function would be required for the multicellularity resulting from the sim mutation, i.e., gl3 would be epistatic to sim. This is in contrast to the additive phenotype seen here (Figs 5B,C,D, 8A,B). However, at least one other R-like gene exists in the Arabidopsis genome whose function probably overlaps in function with that of GL3 (Payne et al., 2000), consistent with the presence of some endoreduplication in gl3 trichomes. Functional redundancy can result in additive phenotypes when mutations acting in the same pathway are combined (Martiensen and Irish, 1999). The phenotype of the sim try double mutant is particularly striking (Figs 5B,E,F, 8C,D). Although the nature of the TRY gene product is unknown, we speculate that TRY may encode Multicellular trichomes in Arabidopsis 3939 a factor that regulates the level of endoreduplication by acting to inhibit endo S phase (Fig. 9). If SIM acts to inhibit mitosis during each endocycle, then the sim mutation would convert the extra rounds of endoreduplication due to the try mutation into additional cells. One problem with this interpretation is that try mutants produce only one additional round of endoreduplication in trichomes, but sim try double mutants appear to produce more than twice as many cells as sim trichomes per TIS (Figs 1B, 3D, 8C,D; Table 1). It is possible that in this double mutant, feedback mechanisms that normally control the degree of endoreduplication are further disrupted. We cannot rule out the possibility that the sim try phenotype results from an interaction with the trichome branching aspect of the try phenotype, or some unknown function of try other than endoreduplication. Given the results of our clonal analysis of sim trichome clusters (Fig. 4), however, it seems unlikely that sim interacts with the proposed cell signaling function of try during trichome initiation. Of course, we do not yet know the molecular or biochemical nature of the SIM gene and its mutation, and thus these genetic interactions must be interpreted with some caution (Martiensen and Irish, 1999). In addition, we only have a single sim allele. The sim phenotype is very recognizable, but despite long-term screening for trichome mutants involving the progeny of tens of thousands of M1 plants, no additional alleles have been recovered. During these screens, multiple alleles of most known trichome genes have been identified. Thus, it is quite possible that this allele is a partial loss-of-function allele, or mutation in very specific domain of the gene product. Nevertheless, the evidence presented here supports a model for the regulation of trichome endoreduplication that is quite consistent with the evidence from other systems indicating that the endocycle is regulated at both the G1/S and G2/M transitions. This dual control of the endo cycle may explain why the sim mutation does not result in trichome ‘tumors’ exhibiting uncontrolled growth. In addition, the sim mutant phenotype may have implications for the evolution of trichome multicellularity. One other example of induction of multicellular trichomes in a plant that normally has unicellular trichomes occurs in cotton fibers, where tissue culture in the presence of auxin and GA can cause developing cotton fibers to divide (Van’t Hof and Saha, 1997). Cotton fiber cells undergo endoreduplication during normal development, similar to the case of Arabidopsis trichomes (Van’t Hof, 1999). Both unicellular and multicellular trichomes are widespread among angiosperms. The sim mutant phenotype suggests that it may be surprisingly easy to switch between unicellular and multicellular trichomes during evolution, and that this switch could be accomplished relatively independent of changes in trichome shape. We wish to thank Ginger Brininstool, Mohamed Noor and Jim Moroney for critical reading of the manuscript; Jolanta Nunley and Olga Borkhsenious for expertise with scanning electron microscopy; Margaret C. Henk and Ron Bouchard for additional technical assistance with microscopy and image production; and Bill Platt, Mohamed Noor and Charles Ramcharan for advice on probability and statistical methods. We also wish to thank two anonymous reviewers for helpful and constructive suggestions. J. C. L.’s research is supported by Grant IBN 9728047 from the National Science Foundation. D. G. O.’s research is supported by Grant GM 5-30753 from the National Institutes of Health. REFERENCES Angchaisuksiri, P., Carlson, P. L., Day, E. B. and Dessypris, E. N. (1994). Replication and endoreduplication in developing megakaryocytes in vitro. Exp. Hematol. 22, 546-550. Barlow, P. (1978). Endopolyploidy: towards an understanding of its biological significance. Acta Biotheor. 27, 1-18. Bell, C. J. and Ecker, J. R. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137-144. Boas, M. L. (1966). Mathematical Methods in the Physical Sciences. J. Wiley & Sons. Chase, R. and Tolloczko, B. (1987). Evidence for differential DNA endoreduplication during the development of a molluscan brain. J. Neurobiol. 18, 395-406. Doerner, P., Jorgensen, J. E., You, R., Steppuhn, J. and Lamb, C. (1996). Control of root growth and development by cyclin expression. Nature 380, 520-523. Fernandez, D., Heck, G., Perry, S., Patterson, S., Bleeker, A. and Fang, S.C. (2000). The embryo MADS domain factor AGL15 acts postembryonically: inhibition of perianth senescence and abscission via constitutive expression. Plant Cell 12, 183-197. Foard, D. (1971). The initial protrusion of a leaf primordium can form without concurrent periclinal cell division. Can. J. Bot. 49, 1601-1603. Foard, D., Haber, A. and Fishman, T. (1965). Initiation of lateral root primordia without completion of mitosis and without cytokinesis in uniseriate pericycle. Am. J. Bot. 52, 580-590. Galbraith, D., Harkins, K. and Knapp, S. (1991). Systemic endopolyploidy in Arabidopsis thaliana. Plant Physiol. 96, 985-989. Gendreau, E., Höft, H., Grandjean, O., Brown, S. and Trass, J. (1998). Phytochrome controls the number of endoreduplication cycles in the Arabidopsis thaliana hypocotyl. Plant J. 13, 221-230. Gendreau, E., Trass, J., Thierry, D., Grandjean, O., Caboche, M. and Höfte, H. (1997). Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 114, 295-305. Grafi, G., Burnett, R. J., Helentjaris, T., Larkins, B. A., DeCaprio, J. A., Sellers, W. R. and Kaelin, W. G. Jr (1996). A maize cDNA encoding a member of the retinoblastoma protein family: involvement in endoreduplication. Proc. Natl. Acad. Sci. USA 93, 8962-8967. Grafi, G. and Larkins, B. A. (1995). Endoreduplication in maize endosperm: involvement of M phase-promoting factor inhibition and induction of S phase-related kinases. Science 269, 1262-1264. Haber, A. (1962). Nonessentiality of concurrent cell divisions for degree of polarization of leaf growth. I. Studies with radiation-induced mitotic inhibition. Am. J. Bot. 49, 583-589. Hayashi, S. (1996). A Cdc2 dependent checkpoint maintains diploidy in Drosophila. Development 122, 1051-1058. Hemerly, A., de Almeida Engler, J., Bergounioux, C., Van Montagu, M., Engler, G., Inze, D. and Ferreira, P. (1995). Dominant negative mutants of the Cdc2 kinase uncouple cell division from iterative plant development. EMBO J. 14, 3925-3936. Hülskamp, M., Miséra, S. and Jürgens, G. (1994). Genetic dissection of trichome cell development in Arabidopsis. Cell 76, 555-566. Hülskamp, M., Schnittger, A. and Folkers, U. (1999). Pattern formation and cell differentiation: trichomes in Arabidopsis as a genetic model system. Int. Rev. Cytol. 186, 147-178. Irish, V. and Sussex, I. (1992). The fate map of the Arabidopsis embryonic shoot apical meristem. Development 115, 745-753. Kaplan, D. and Hagemann, W. (1991). The relationship of cell and organism in vascular plants. Bioscience 41, 693-703. Katzen, A. L., Jackson, J., Harmon, B. P., Fung, S.-M., Ramsey, G. and Bishop, J. M. (1998). Drosophila myb is required for the G2/M transition and maintenance of diploidy. Genes Dev. 12, 831-843. Knoblich, J., Sauer, K., Jones, L., Richardson, H., Saint, R. and Lehner, C. (1994). Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77, 107-120. Konieczny, A. and Ausubel, F. (1993). A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4, 403-410. Koornneef, M., Dellaert, S. W. M. and van der Veen, J. H. (1982). EMSand radiation-induced mutation frequencies at individual loci in Arabidopsis thaliana (L) Heynh. Mutation Res. 93, 109-123. Kowles, R. and Phillips, R. (1985). DNA amplification patterns in maize 3940 J. D. Walker and others endosperm nuclei during kernel development. Proc. Natl. Acad. Sci. USA 82, 7010-7014. Larkin, J., Walker, J., Bolognesi-Winfield, A., Gray, J. and Walker, A. (1999). Allele-specific interactions between ttg and gl1 during trichome development in Arabidopsis thaliana. Genetics 151, 1591-1604. Larkin, J. C., Oppenheimer, D. G., Lloyd, A., Paparozzi, E. T. and Marks, M. D. (1994). The roles of GLABROUS1 and TRANSPARENT TESTA GLABRA genes in Arabidopsis trichome development. Plant Cell 6, 10651076. Larkin, J. C., Young, N., Prigge, M. and Marks, M. D. (1996). The control of trichome spacing and number in Arabidopsis. Development 122, 9971005. Lawson, E. J. R., Scofield, S. R., Sjodin, C., Jones, J. D. G. and Dean, C. (1994). Modification of the 5′ untranslated leader region of the maize Activator element leads to increased activity in Arabidopsis. Mol. Gen. Genet. 245, 608-615. Marks, M. D. (1997). Molecular genetic analysis of trichome development in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 137163. Martiensen, R. and Irish, V. (1999). Copying out our ABCs: the role of gene redundancy in interpreting genetic hierarchies. Trends Genet. 15, 435-437. McDaniel, C. and Poethig, R. (1988). Cell-lineage patterns in the shoot apical meristem of the germinating maize embryo. Planta 175, 13-22. Melaragno, J., Mehrota, B. and Coleman, A. (1993). Relationship between endoploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5, 1661-1668. Nagl, W. (1976). DNA endoreduplication and polyteny understood as evolutionary strategies. Nature 261, 614-615. Luo, D. and Oppenheimer, D. G. (1999). Genetic control of trichome branch number in Arabidopsis: the roles of the FURCA loci. Development 126, 5547-5557. Orr-Weaver, T. (1994). Developmental modification of the Drosophila cell cycle. Trends Genet. 10, 321-327. Payne, C. T., Zhang, F. and Lloyd, A. M. (2000). GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics (in press). Poethig, R. and Sussex, I. (1985). The cellular parameters of leaf development in tobacco: a clonal analysis. Planta 165, 170-184. Reynolds, J., Eisses, J. and Sylvester, A. (1998). Balancing division and expansion during the maize leaf morphogenesis: analysis of the mutant, warty-1. Development 125, 259-268. Rice, W. (1989). Analyzing tables of statistical tests. Evolution 43, 223-225. Ruzin, S. (1999). Plant Microtechnique and Microscopy. Oxford University Press. Sarto, G. E., Stubblefield, P. A. and Therman, E. (1982). Endomitosis in human trophoblast. Hum. Genet. 62, 228-232. Sauer, K., Knoblich, J. A., Richardson, H. and Lehner, C. F. (1995). Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Schnittger, A., Folkers, U., Schwab, B., Jürgens, G. and Hülskamp, M. (1999). Generation of a spacing pattern: the role of TRIPTYCHON in trichome patterning in Arabidopsis. Plant Cell 11, 1105-1116. Schnittger, A., Jürgens, G. and Hülskamp, M. (1998). Tissue layer and organ specificity of trichome formation are regulated by GLABRA1 and TRIPTYCHON in Arabidopsis. Development 125, 2283-2289. Segers, G., Gadisseur, I., Bergounioux, C., de Ameilda-Engler, J., Jacqmard, A., van Montagu, M. and Inzé, D. (1996). The Arabidopsis cyclin-dependent kinase gene cdc2bAT is preferentially expressed during S and G2 phases of the cell cycle. Plant J. 10, 601-612. Sigrist, S. J. and Lehner, C. F. (1997). Drosophila fizzy-related downregulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90, 671-681. Smith, A. V. and Orr-Weaver, T. L. (1991). The regulation of the cell cycle during Drosophila embryogenesis: the transition to polyteny. Development 112, 997-1008. Smith, L., Hake, S. and Sylvester, A. (1996). The tangled-1 mutation alters cell division orientations throughout maize leaf development without altering leaf shape. Development 122, 481-489. Steeves, T. A. and Sussex, I. M. (1989). Patterns in Plant Development. Cambridge University Press. Szymanski, D. and Marks, M. (1998). GLABROUS1 overexpression and TRIPTYCHON alter the cell cycle and trichome cell fate in Arabidopsis. Plant Cell 10, 2047-2062. Szymanski, D. B., Jilk, R. A., Pollock, S. M. and Marks, M. D. (1998). Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125, 1161-1171. Torres-Ruiz, R. and Jürgens, G. (1994). Mutations in the FASS gene uncouple pattern formation in Arabidopsis development. Development 120, 2967-2978. Traas, J., Bellini, C., Nacry, P., Kronenberger, J., Bouchez, D. and Caboche, M. (1995). Normal differentiation patterns in plants lacking microtubular preprophase bands. Nature 375, 676-677. Traas, J., Hülskamp, M., Gendreau, E. and Höfte, H. (1998). Endoreduplication and development: rule without dividing? Curr. Opin. Plant Biol. 1, 498-503. Van’t Hof, J. (1999). Increased nuclear DNA content in developing cotton fiber cells. Am. J. Bot. 86, 776-779. Van’t Hof, J. and Saha, S. (1997). Cotton fibers can undergo cell division. Am.. J. Bot. 84, 1231-1235.