* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ATP Biochemistry: The Chemical Composition of Living Matter
Survey
Document related concepts
Vectors in gene therapy wikipedia , lookup
Protein purification wikipedia , lookup
Photosynthesis wikipedia , lookup
Protein–protein interaction wikipedia , lookup
DNA-encoded chemical library wikipedia , lookup
Carbohydrate wikipedia , lookup
Protein adsorption wikipedia , lookup
Chemical biology wikipedia , lookup
List of types of proteins wikipedia , lookup
History of molecular biology wikipedia , lookup
Hypothetical types of biochemistry wikipedia , lookup
Biomolecular engineering wikipedia , lookup
Abiogenesis wikipedia , lookup
Animal nutrition wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
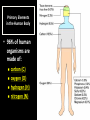
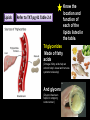
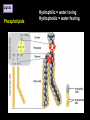
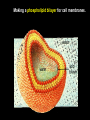
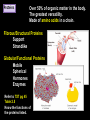
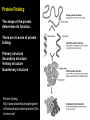
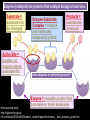
CHAPTER 2 (Basic Chemistry) Biochemistry: The chemical composition of living matter www.pbs.org Biochemistry: The Chemical Composition of Living Matter Steroids Starch Enzymes Water HCl s293.photobucket.com kau.edu.sa NaOH Lipids www.mentalfloss.com alibaba.com Glycogen bioinformatics.sussex.ac.uk Buffers ATP sportsandexercisephysiology.com education.vetmed.vt.edu www.peepresearch.org i.ehow.com Proteins DNA vis.lbl.gov Electrolytes (salts) www.wired.com Sugars hermann-uwe.de www.techjackal.net www.all-about-forensic-science.com Biochemistry: The Chemical Composition of Living Matter ORGANIC CHEMICALS ARE BASED ON CARBON MOLECULES. High heat capacity Takes a lot of heat to change the temperature. Water Chemical Reactivity Can easily react with certain chemicals. (like foods) Cushioning Ability Absorbs shockwaves. No Carbon = INORGANIC Polarity Solvent Can dissolve many other chemicals (like oxygen, salts, glucose, wastes, etc.). Biochemistry: The Chemical Composition of Living Matter ORGANIC CHEMICALS ARE BASED ON CARBON MOLECULES. Contain calcium and phosphorus Makes strong bones and teeth. Used in body functions Salts are required for nerve impulses. Iron is required for hemoglobin to transport oxygen to cells. Salts No Carbon = INORGANIC Electrolytes Conducts electrical currents in solutions. Dissociation Can dissolve into ions in body fluids with the pull of the polarity of water. Biochemistry: The Chemical Composition of Living Matter ORGANIC CHEMICALS ARE BASED ON CARBON MOLECULES. ACIDS BASES Acids and Bases Taste SOUR. Taste BITTER. Can dissolve some Can dissolve some substances. substances. No Carbon = Release H+ Release OHINORGANIC (hydrogen ions) (hydroxyl ions) when when dissolved in dissolved in water. Electrolytes water. Proton donor. Proton acceptor. (just like salt!) If an Acid releases all If a Base releases all Conducts electrical of its H+ ions it is a of its OH- ions it is a currents in solutions. STRONG acid. STRONG base. See Table 2.11 TXT pg If it only releases If it only releases 38. some H+ ions it is a some OH- ions it is a WEAK acid. WEAK base. Biochemistry: The Chemical Composition of Living Matter ORGANIC CHEMICALS ARE BASED ON CARBON MOLECULES. ACIDS BASES Acids and Bases Taste SOUR. Taste BITTER. Can dissolve some Can dissolve some substances. substances. No Carbon = Release H+ Release OHINORGANIC (hydrogen ions) (hydroxyl ions) when when dissolved in dissolved in water. water. Proton donor. Proton acceptor. If an Acid releases all NEUTRALIZATION If a Base releases all REACTION! of its H+ ions it is a of its OH- ions it is a STRONG acid. STRONG base. If it only releases If it only releases some H+ ions it is a some OH- ions it is a WEAK acid. WEAK base. Biochemistry: The Chemical Composition of Living Matter ORGANIC CHEMICALS ARE BASED ON CARBON MOLECULES. High amount of H+ ions. Acids and Bases No Carbon = INORGANIC At what pH is there equal amounts of H+ and OH- ions? Low amount of H+ ions. pH scale – Based on amount of H+ ions in the solution. Biochemistry: The Chemical Composition of Living Matter Steroids Starch Enzymes Water HCl s293.photobucket.com kau.edu.sa NaOH Lipids www.mentalfloss.com alibaba.com Glycogen bioinformatics.sussex.ac.uk Buffers ATP sportsandexercisephysiology.com education.vetmed.vt.edu www.peepresearch.org i.ehow.com Proteins DNA vis.lbl.gov www.wired.com Sugars hermann-uwe.de Electrolytes (salts) WHICH ARE ORGANIC?? LET’S LEARN A LITTLE MORE… www.all-about-forensic-science.com www.techjackal.net Primary Elements in the Human Body • 96% of human organisms are made of: carbon (C) oxygen (O) hydrogen (H) nitrogen (N) MAKING AND BREAKING MOLECULES • Synthesis – building bigger molecules from smaller molecules – building cells & bodies • repair • growth • reproduction • Digestion – taking big molecules apart – getting raw materials • for synthesis & growth – making energy (ATP) • for synthesis, growth & everyday functions Synthesis = building molecules 2 monosaccharides | Glucose 1 sugar | Fructose 1 sugar 1 disaccharide | Sucrose 2 sugars linked (table sugar) This is how plants build sugars that we eat. Hydrolysis = splitting with water 1 disaccharide | Sucrose 2 sugars linked (table sugar) 2 monosaccharides | Glucose 1 sugar | Fructose 1 sugar This is how we digest the sugars that we eat. Lipids Refer to TXT pg 41 Table 2.4 Know the location and function of each of the lipids listed in the table. Triglycerides Made of fatty acids (Omega-3 fatty acids help an unborn baby’s visual and nervous systems to develop) And glycerol (Glycerol has been helpful in stopping some cancer.) Lipids Phospholipids Hydrophilic = water loving Hydrophobic = water fearing Making a phospholipid bilayer for cell membranes. Lipids Steroids Total Cholesterol Levels Need to be below 200 LDL = BAD! Needs to be below 100 HDL = GOOD!! Needs to be 60 or above Triglycerides = BAD! Needs to be 150 or below Cholesterol Quiz http://www.americanheart.org/presenter.jhtml?identifier=3032767 Cholesterol Video 23:00 http://player.discoveryeducation.com/index.cfm?guidAssetId=D0DC3225-D27A-4E3A8CD0-7BCB3B1241A4&blnFromSearch=1&productcode=US Proteins Over 50% of organic matter in the body. The greatest versatility. Made of amino acids in a chain. Fibrous/Structural Proteins Support Strandlike Globular/Functional Proteins Mobile Spherical Hormones Enzymes Refer to TXT pg 45 Table 2.5 Know the functions of the proteins listed. www.ucl.ac.uk Protein Folding The shape of the protein determines its function. There are 4 Levels of protein folding: Primary structure Secondary structure Tertiary structure Quarternary structure Protein folding http://www.stolaf.edu/people/gianni ni/flashanimat/proteins/protein%20s tructure.swf Enzymes (catalysts) are proteins that catalyze biological reactions. Substrate = substance to be changed Active Site = location on enzyme where substrate fits Enzyme-Substrate Complex = enzyme and molecules temporarily joined Products = substances produced Is this a digestive or synthesizing enzyme? Enzyme = reusable protein that can make or break molecules How enzymes work: http://highered.mcgrawhill.com/sites/0072495855/student_view0/chapter2/animation__how_enzymes_work.html Denaturation of Protein http://highered.mcgraw-hill.com/sites/0072507470/student_view0/chapter2/animation__protein_denaturation.html Nucleic Acids are DNA nucleotide chains – nucleotides chained into a polymer • DNA –double-sided –double helix –A, C, G, T • RNA –single-sided –A, C, G, U RNA What is the difference between DNA & Genes & Chromosomes? DNA folds into chromosomes. A gene is a section of a chromosome that controls the making of a specific protein. gene DNA folding to make Chromosomes 2:21 http://www.cells.de/cellseng/1medienarchiv/Zellstruktur/Zellkern/DNA_condensation/Flash__C13105.htm Assignments: TXT p51 Multiple Choice #1-9 (some answers are not in the notes.) TXT p52 At the Clinic (select one question (out of #1-4) to answer.)