* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 19_Glycolysis, aerobic oxidation of glucose
Multi-state modeling of biomolecules wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Cryobiology wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biosynthesis wikipedia , lookup
Biochemical cascade wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Blood sugar level wikipedia , lookup
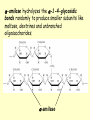
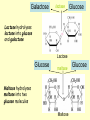
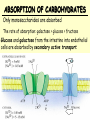
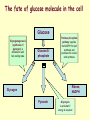
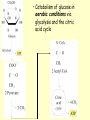
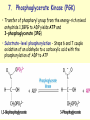
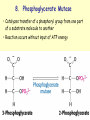
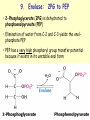
METABOLISM OF CARBOHYDRATES: GLYCOLYSIS For centuries, bakeries and breweries have exploited the conversion of glucose to ethanol and CO2 by glycolysis in yeast DIGESTION OF CARBOHYDRATES Glycogen, starch and disaccharides (sucrose, lactose and maltose) are hydrolyzed to monosaccharide units in the gastrointestinal tract. The process of digestion starts in the mouth by the salivary enzyme –amilase. The time for digestion in mouth is limited. Salivary -amilase is inhibited in stomach due to the action of hydrochloric acid. Another -amilase is produced in pancreas and is available in the intestine. -amilase hydrolyzes the -1-4-glycosidic bonds randomly to produce smaller subunits like maltose, dextrines and unbranched oligosaccharides. -amilase The intestinal juice contains enzymes hydrolyzing disaccharides into monosaccharides (they are produced in the intestinal wall) Sucrase hydrolyses sucrose into glucose and fructose Glucose sucrase Fructose Sucrose Galactose lactase Glucose Lactase hydrolyses lactose into glucose and galactose Lactose Glucose maltase Maltase hydrolyses maltose into two glucose molecules Maltose Glucose ABSORPTION OF CARBOHYDRATES Only monosaccharides are absorbed The rate of absorption: galactose > glucose > fructose Glucose and galactose from the intestine into endothelial cells are absorbed by secondary active transport Na+ Protein Glucose Protein Carrier protein is specific for D-glucose or D-galactose. L-forms are not transported. There are competition between glucose and galactose for the same carrier molecule; thus glucose can inhibit absorption of galactose. Fructose is absorbed from intestine into intestinal cells by facilitated diffusion. Absorption of glucose from intestinal cells into bloodstream is by facilitated diffusion. Transport of glucose from blood into cells of different organs is mainly by facilitated diffusion. The protein facilitating the glucose transport is called glucose transporter (GluT). GluT are of 5 types. GluT2 is located mainly in hepatocytes membranes (it transport glucose into cells when blood sugar is high); GluT1 is seen in erythrocytes and endothelial cells; GluT3 is located in neuronal cells (has higher affinity to glucose); GluT5 – in intestine and kidneys; GluT4 - in muscles and fat cells. The fate of glucose molecule in the cell Glucose Glycogenogenesis (synthesis of glycogen) is activated in well fed, resting state Glucose-6phosphate Pentose phosphate pathway supplies the NADPH for lipid synthesis and pentoses for nucleic acid synthesis Ribose, NADPH Glycogen Pyruvate Glycolysis is activated if energy is required Glycolysis is the earliest discovered and most important process of carbohydrates metabolism. Glycolysis – metabolic pathway in which glucose is transformed to pyruvate with production of a small amount of energy in the form of ATP or NADH. Glycolysis is an anaerobic process (it does not require oxygen). Glycolysis pathway is used by anaerobic as well as aerobic organisms. In glycolysis one molecule of glucose is converted into two molecules of pyruvate. In eukaryotic cells, glycolysis takes place in the cytosol. Pyruvate can be further metabolized to: (1) Lactate or ethanol (anaerobic conditions) (2) Acetyl CoA (aerobic conditions) • Acetyl CoA is further oxidized to CO2 and H2O via the citric acid cycle • Much more ATP is generated from the citric acid cycle than from glycolysis Acetyl CoA • Catabolism of glucose in aerobic conditions via glycolysis and the citric acid cycle The glycolytic pathway consist of ten enzymecatalyzed reactions that begin with a glucose and split it into two molecules of pyruvate Glycolysis (10 reactions) can be divided into three stages • In the 1st stage (hexose stage) 2 ATP are consumed per glucose • In the 3rd stage (triose stage) 4 ATP are produced per glucose • Net: 2 ATP produced per glucose Stage 1, which is the conversion of glucose into fructose 1,6-bisphosphate, consists of three steps: a phosphorylation, an isomerization, and a second phosphorylation reaction. The strategy of these initial steps in glycolysis is to trap the glucose in the cell and form a compound that can be readily cleaved into phosphorylated threecarbon units. Stage 2 is the cleavage of the fructose 1,6-bisphosphate into two three-carbon fragments dihydroxyacetone phosphate and glyceraldehyde 3phosphate. Dihydroxyacetone phosphate and glyceraldehyde 3phosphate are readily interconvertible. In stage 3, ATP is harvested when the threecarbon fragments are oxidized to pyruvate. Glycolysis Has 10 Enzyme-Catalyzed Steps • Each chemical reaction prepares a substrate for the next step in the process 1. Hexokinase • Transfers the g-phosphoryl of ATP to glucose C-6 oxygen to generate glucose 6-phosphate (G6P) • Four kinases in glycolysis: steps 1,3,7, and 10 • All four kinases require Mg2+ and have a similar mechanism Properties of hexokinases • Broad substrate specificity - hexokinases can phosphorylate glucose, mannose and fructose • Isozymes - multiple forms of hexokinase occur in mammalian tissues and yeast • Hexokinases I, II, III are active at normal glucose concentrations • Hexokinase IV (Glucokinase) is active at higher glucose levels, allows the liver to respond to large increases in blood glucose • Hexokinases I, II and III are allosterically inhibited by physiological concentrations of their immediate product, glucose-6-phosphate, but glucokinase is not. 2. Glucose 6-Phosphate Isomerase • Converts glucose 6-phosphate (G6P) (an aldose) to fructose 6-phosphate (F6P) (a ketose) • Enzyme preferentially binds the a-anomer of G6P (converts to open chain form in the active site) • Enzyme is highly stereospecific for G6P and F6P • Isomerase reaction is near-equilibrium in cells 3. Phosphofructokinase-1 (PFK-1) • Catalyzes transfer of a phosphoryl group from ATP to the C-1 hydroxyl group of F6P to form fructose 1,6bisphosphate (F1,6BP) • PFK-1 is metabolically irreversible and a critical regulatory point for glycolysis in most cells • A second phosphofructokinase (PFK-2) synthesizes fructose 2,6-bisphosphate (F2,6BP) 4. Aldolase • Aldolase cleaves the hexose F1,6BP into two triose phosphates: glyceraldehyde 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP) • Reaction is near-equilibrium, not a control point 5. Triose Phosphate Isomerase (TPI) • Conversion of DHAP into GAP • Reaction is very fast, only the D-isomer of GAP is formed • Reaction is reversible. At equilibrium, 96% of the triose phosphate is DHAP. However, the reaction proceeds readily from DHAP to GAP because the subsequent reactions of glycolysis remove this product. Fate of carbon atoms from hexose stage to triose stage 6. Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) • Conversion of GAP to 1,3-bisphosphoglycerate (1,3BPG) • Molecule of NAD+ is reduced to NADH • Energy from oxidation of GAP is conserved in acidanhydride linkage of 1,3BPG • Next step of glycolysis uses the high-energy phosphate of 1,3BPG to form ATP from ADP 7. Phosphoglycerate Kinase (PGK) • Transfer of phosphoryl group from the energy-rich mixed anhydride 1,3BPG to ADP yields ATP and 3-phosphoglycerate (3PG) • Substrate-level phosphorylation - Steps 6 and 7 couple oxidation of an aldehyde to a carboxylic acid with the phosphorylation of ADP to ATP 8. Phosphoglycerate Mutase • Catalyzes transfer of a phosphoryl group from one part of a substrate molecule to another • Reaction occurs without input of ATP energy 9. Enolase: 2PG to PEP • 2-Phosphoglycerate (2PG) is dehydrated to phosphoenolpyruvate (PEP) • Elimination of water from C-2 and C-3 yields the enolphosphate PEP • PEP has a very high phosphoryl group transfer potential because it exists in its unstable enol form 10. Pyruvate Kinase (PK) PEP + ADP Pyruvate + ATP • Catalyzes a substrate-level phosphorylation • Metabolically irreversible reaction • Regulation both by allosteric modulators and by covalent modification • Pyruvate kinase gene can be regulated by various hormones and nutrients Net reaction of glycolysis During the convertion of glucose to pyruvate: • Two molecules of ATP are produced • Two molecules of NAD+ are reduced to NADH Glucose + 2 ADP + 2 NAD+ + 2 Pi 2 Pyruvate + 2 ATP + 2 NADH + 2 H+ + 2 H2O