* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry 250A -- Exam #3 Answer Key -
Survey
Document related concepts
Woodward–Hoffmann rules wikipedia , lookup
Ene reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Stille reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Transcript
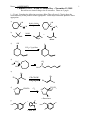
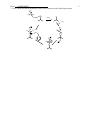
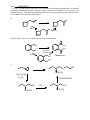
Name: ANSWER KEY 1 Chemistry 250A -- Exam #3 Answer Key -- November 13, 2009 Show non-zero formal charges for all structures. There are 5 pages. 1. (21 pts) Complete the following reactions. (Hint: They all react!) Clearly show the stereochemistry of the products where appropriate. Label major and minor products where appropriate. a) OH C OH HgSO4, H2SO4 H 2O CH C CH3 O b) NaOH + Br major c) minor OH O CrO3 • 2 pyridine d) H3C HO CH3 N e) N Δ H 3C H 3C Br Ph Ph CH3CH2OK Ph Ph CH3CH2OH Br Br f) O CH2CH3 Cl2 O CH2CH3 Cl Cl + Enantiomer g) Cl Cl 1. O NK O 2. KOH, H2O Cl H2N Name: ANSWER KEY 2 2. (14 pts) When the alkene shown below is treated with borane in THF, followed by H2O2 and NaOH, two products, A and B are formed in at 57:43 ratio. When disiamylborane is used instead, the ratio of A to B is 97:3. 1. BH3, THF 2. H2O2, NaOH 1. disiamylborane 2. H2O2, NaOH 57% A: 43% B 97% A: 3% B a) Provide names for both the alkene shown above and for H2O2. Trans-(or E)-4-methyl-2-pentene Hydrogen peroxide b) Draw the structure of disiamylborane and THF. H O B c) Draw the structures of A and B. (Clearly indicate which is which.) OH A B OH d) Clearly explain the reason for the differences in the percentages. Boron and hydrogen add across the double bond simultaneously. Since the boron is larger (and hence more sterically demanding) than a hydrogen, it prefers to add to the least hindered side. But since the boron in BH3 is attached to small groups (hydrogens), this steric preference isn’t to dramatic. However, when two of the hydrogens on the boron are replaced with bulky disiamyl groups, the boron end of the B-H bond has a much stronger preference for the least hindered position. Essentially, when the boron adds to the left hand side of the double bond the disiamyl groups run into the isopropyl group attached there, whereas there is much less steric hindrance with the methyl group on the other side. (In the second step of the reaction the boron is replaced with an OH, so the major product has the OH on the least hindered side.) Name: ANSWER KEY 3. (9 pts) Write a detailed mechanism (using curved arrows) for the following reaction. O S Cl Cl OH Cl SOCl2 pyridine SO2 O O Cl S Cl O H Cl S O N O Cl S O Cl Cl 3 Name: ANSWER KEY 4. (10 pts) Rank the following compounds according to the indicated property: a) stability C A D B D most stable C > > B > A least stable b) Ratio of substitution/elimination Br OTs CH3CH2ONa CH3CH2NH2 B A Br H 3C NaSH OTs NaOH C C most substitution D A > > B > D most elimination 5. (10 pts) Write a detailed mechanism (using curved arrows) to account for the products observed in the following reaction. CH3 CH3 H3 PO4 OH CH3 CH3 + CH3 CH3 CH3 CH3 OH2 + + CH3 CH3 + H CH3 CH3 H2 PO4 - CH3 + H H2 PO4 - CH3 4 Name: ANSWER KEY 6. (16 pts) When menthyl chloride is treated with sodium ethoxide in ethanol, only one elimination product is produced. However when menthyl chloride is treated with ethanol in water two elimination products are formed. 5 CH3CH2ONa CH3CH2OH Cl CH3CH2OH Menthyl chloride H2O 100% 0% 32% 68% a) Draw detailed mechanisms (using curved arrows) for both of these reactions showing the formation of the products. Then clearly explain why only one product is obtained under the first set of conditions, and why a mixture favoring the other product is obtained under the second set of conditions. CH3CH2O H H Cl H CH3CH2ONa CH3CH2OH Not anti to leaving group In the presence of a strong base, the first reaction proceeds via an E2 mechanism as shown above. The E2 reaction proceeds fastest when the bonds to the hydrogen being abstracted and the leaving group are periplanar to each other, which in a six-membered ring requires that they be in a trans-diaxial conformation. In menthyl chloride there is only one beta hydrogen in this orientation and therefore elimination proceeds to give only the product shown. By contrast, the second reaction proceeds via an E1 mechanism as shown below. (The absence of a strong base makes the E2 mechanism unfavorable.) In the E1 mechanism the leaving group leaves first, producing a carbocation. The hydrogen is removed in a second step, but since the leaving group has already left, there is no requirement for the hydrogen to be anti to the leaving group. Thus, unlike the E2 case, both isomeric products can be efficiently produced. The second product, with the isopropyl group on the double bond, contains a more highly substituted double bond and thus is more stable than the first product and, to the extent that the transition state also has double bond character, is produced to a larger extent. Cl H H CH3CH2OH H 2O H Menthyl chloride H ROH 32% 68% More highly substituted alkene is more stable. Name: ANSWER KEY 6 7. (20 pts) List the reagents necessary to accomplish the following transformations. In addition to the indicated starting material, you may use any molecules of 3 carbons or less as well as any standard reagents. (For multistep transformations show the major organic product for each step. You do not need to show the mechanisms.) a) N3 HBr NaN3 Br b) Extra credit (2 pts) if you can provide the name of the product. O OH Cl OH Cl Na2Cr2O7 H2SO4, H2O NaOH OH O Phthalic Acid OH c) S O OCH2CH3 CH3CH2OH H2SO4 CH3CH2CH2SNa OH OCH2CH3 TsCl pyridine OTs OCH2CH3