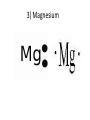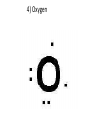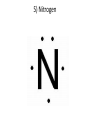* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1.5.16(Chem) - mrcarlsonschemistryclass
Metastable inner-shell molecular state wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Double layer forces wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Metallic bonding wikipedia , lookup
Coordination complex wikipedia , lookup
Electronegativity wikipedia , lookup
Electron configuration wikipedia , lookup
Electric charge wikipedia , lookup
Sodium hydroxide wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Magnesium in biology wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
History of molecular theory wikipedia , lookup
Sodium bicarbonate wikipedia , lookup
Oxidation state wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Electrochemistry wikipedia , lookup
Bond valence method wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical bond wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Metalloprotein wikipedia , lookup
Ionic compound wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
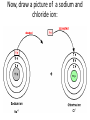
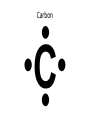
1) BellRinger: Remind – Read article and then do a Sentence Phrase Word 2) Worksheet #26 3)QUIZ #9 Draw Picture of a sodium and chlorine atom: Now, draw a picture of a sodium and chloride ion: Cations and Anions • Cations are ions with a POSITIVE charge. • Anions are ions with a NEGATIVE charge. • Draw the funny way to remember cations and anions: • Compounds • Atoms bonded together with an IONIC bond are called ionic compounds. • An ionic bond is a METAL bonded with a NONMETAL. • Draw the crystal lattice structure for sodium chloride: • Empirical Formula • Refers to the ratio of ions in a compound • NaCl has a ratio of 1:1 (1 to 1). • MgCl2 has a ratio of 1:2 (1 to 2). • AlCl3 has a ratio of 1:3 (1 to 3). Lewis Dot Structures Dots used to represent VALENCE electrons around an atom. Lewis Dot Structures • Sodium 2) Chlorine 3) Magnesium 4) Oxygen 5) Nitrogen Carbon Oxidation Number • Charge that an atom will have if made into an ion. • Na becomes Na+, so its oxidation 1+ number is . • Cl becomes Cl-, so its oxidation number is 1- . Magnesium has 2 valence electrons Magnesium has a 2+ charge Flourine has 7 valence electrons Flourine has a 1- charge Mg (2+) MgF2 F (-1) F(-1) Ionic Combinations • Sodium oxide (Sodium and oxygen) Na (+1) Na(+1) Na2O O(2-) MgO Mg (2+) Cancel out perfectly O (2-) Criss -Cross Method • Lithium oxide REACTION OF SODIUM AND CHLORINE • Video IONISATION • Video QUIZ #9 1)Draw the Lewis dot structure for Aluminum 2) Give the chemical formula for lithium and fluorine coming together.