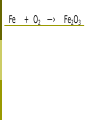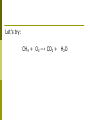* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Reactions and Equations
Chemical industry wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Isotopic labeling wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Asymmetric induction wikipedia , lookup
Atomic theory wikipedia , lookup
Marcus theory wikipedia , lookup
History of chemistry wikipedia , lookup
Acid–base reaction wikipedia , lookup
Electrolysis of water wikipedia , lookup
Drug discovery wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Photosynthesis wikipedia , lookup
Organic chemistry wikipedia , lookup
Process chemistry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Rate equation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Electrochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Click chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Transition state theory wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Today-- Welcome Back! • • • • Turn in “Chapter 6 Extra Credit” Unexpected Changes Lab Introduce Chapter 9 (LAST CHAPTER) Outlining – NOPE! Chapter 9 – Chemical Reactions and Equations Learning Objectives: ■ Be able to balance chemical equations by applying the law of conservation of mass. ■ Be able to recognize synthesis, decomposition, single replacement, double replacement, combustion and neutralization reactions. Law of Conservation of Matter • Conservation of Matter: in all chemical and physical changes, matter is neither created or destroyed ■ • The total mass in a chemical reaction remains constant Antoine Lavoisier: ■ Made accurate and precise measurements during chemical reactions Reactants ―› Products • Reactants: the substances that enter into the reaction • Products: the substances that are produced by the reaction Why Do Reactions Occur • Think back to what we know about atoms and specifically their electrons? • Through chemical reactions, atoms have the opportunity to obtain complete sets of valence electrons and thus become more stable. Five General Types of Chemical Reactions • • Direct Combination (Synthesis) • • • • Decomposition Single-Replacement Double-Replacement Combustion By knowing the type of reaction that is occurring, you can predict the products that will be formed. Chemical Equations • A method of describing chemical reactions ■ Word Equations • ■ Calcium + Oxygen ―› Calcium Oxide Formula Equations • 2Ca + O2 ―› 2 CaO the arrow → separates the reactants from the products Completing the Chemical Equation • Complete the chemical equation by describing the physical state of each substance: ■ ■ ■ ■ Solid (s) Liquid (l) Gas (g) Aqueous (aq) means dissolved in water Symbols used in equations • • Double arrow reaction ∆ reaction • Pt indicates a reversible shows that heat is supplied to the is used to indicate a catalyst is supplied, in this case, platinum. What is a catalyst? • A substance that speeds up a reaction, without being changed or used up by the reaction. • Enzymes are biological catalysts. • How can you physically speed up a reaction? I. Direct Combination Reactions (also called synthesis reactions). General form: A + B → AB (two reactants make a single product) A, B = elements or compounds AB = compound consisting of A and B ■ This is the only type of chemical reaction in which there is a single product formed. This single product is always more complex than the reactants. Examples of Synthesis Reactions ■ ■ calcium + oxygen yields calcium oxide 2Ca + O2 → 2CaO ■ ■ Notice: All equations show two (or more) reactants, but only one product. http://www.ric.edu/ptiskus/reactions/Index.htm II. Decomposition Reactions General form: AB → A + B (one reactant makes two or more products) AB = compound A, B = elements or simpler compounds ▪ This is the only type of chemical reaction in which there is a single reactant. This single reactant is always more complex than the products. Decomposition Reactions: Examples ■ water yields hydrogen and oxygen 2H2O ■ ■ 2H2 + O2 marble (calcium carbonate) yields calcium oxide and carbon dioxide CaCO3 ■ → → CaO + CO2 Notice: all equations show a single reactant decomposing into two (or more) products. http://www.ric.edu/ptiskus/reactions/Index.htm Balancing Chemical Equations • The Law of Conservation of Matter states that: ■ Matter is neither created nor destroyed! ■ For mass to remain constant both before and after a reaction, the number of atoms must remain constant Step 1: Balancing Equations • Write the word equation that describes the reaction. iron + oxygen ―› iron oxide Step 2: Balancing Equations 2. Replace the words in the equation with symbols and formulas. Fe + O2 ―› Fe2O3 Do we have the same numbers of each atoms on both sides of arrow? Does this follow the law of conservation of matter? Step 3: Balancing Equations 3. Count the # of atoms of each element on both sides of the equation. Fe + O2 ―› Fe2O3 Step 4: Balancing Equations 4. Starting with elements that only occur in one substance on each side of the equation, make sure that each side of the equation has an equal # of that element. Proceed with all elements. Remember that changing the # of one element may alter elements that have already been balanced. Fe + O2 ―› Fe2O3 Let’s try: CH4 + O2 ―› CO2 + H2O Never • Never change a subscript to balance an equation. If you change the formula you are describing a different reaction. ■ H2O is a different compound than H2O2 ■ • Never put a coefficient in the middle of a formula ■ 2 NaCl is okay, Na2Cl is not. Balancing Equations: Examples ■ H2 ■ Co + ■ ■ + O2 → O2 → Co2O3 Pb(NO3)2 + K2S → C2H6 + O2 → H2O PbS + H2O + CO2 KNO3 Balance the following iron(II) chloride + sodium phosphate → sodium chloride + iron (II) phosphate FeCl2 + Na3PO4 → NaCl + Fe3(PO4)2 Today ■ ■ Look at Single-Replacement Reactions. Begin “Single-Replacement Lab” set-up. Single-Replacement Reactions ■ Copper metal and silver nitrate: Cu(s) + AgNO3(aq) → Ag(s) + CuNO3(aq) ■ ■ ■ What do you observe about the reaction? What do you notice about the chemical equation? Cu must be more reactive than Ag in order for the reaction to take place. Single-Replacement Reactions General Form: A + BX → AX + B One element and one compound recombine (switch partners) AX, BX = ionic compounds A, B = Metals X = ion that switches partners *Metal ‘A’ must be more reactive than ‘B’ for this to occur Single-Replacement Lab Today you will do the following: 1. Formulate a question for the lab 2. Formulate a hypothesis 3. Design procedures 4. Create a data table. IV. Double-Replacement Reactions General form: AX + BY → AY + BX (Positive ions in two compounds are exchanged) A,B = positive ions X,Y = negative ions ■ This is the only type of chemical reaction with two compounds as reactants and two compounds as products. Double Replacement Examples ■ calcium carbonate and hydrochloric acid yield calcium chloride and carbonic acid CaCO3 + 2HCl → CaCl2 + H2CO3 ■ Notice: in this reaction, two ionic compounds exchange ions to form two new ionic compounds www.ric.edu/ptiskus/reactions/Index.htm IV. Double-Replacement Reactions General form: AX + BY → AY + BX (Positive ions in two compounds are exchanged) A,B = positive ions X,Y = negative ions ■ This is the only type of chemical reaction with two compounds as reactants and two compounds as products. Double Replacement Examples ■ calcium carbonate and hydrochloric acid yield calcium chloride and carbonic acid CaCO3 + 2HCl → CaCl2 + H2CO3 ■ Notice: in this reaction, two ionic compounds exchange ions to form two new ionic compounds www.ric.edu/ptiskus/reactions/Index.htm Rules of Double-Replacement Reactions ■ ■ Reactants must be dissolved in water (releasing the ions). Will occur if one of the products : • • • is a molecule (covalent), a precipitate (solid comes out of solution), or an insoluble gas. V. Combustion Reactions General Form: CxHy + O2 → H2O + CO2 (hydrocarbon and oxygen react to form carbon dioxide and water) ■ This is the only type of chemical reaction where something reacts with oxygen and forms carbon dioxide and water Combustion Examples ▪ Methane reacts with oxygen: CH4 (methane) + O2 → H2O + CO2 ▪ Gasohol reacts with oxygen: C2H5OH (ethanol) + O2 → H2O + CO2 ▪ Notice: in both cases, water and carbon dioxide are the products. www.ric.edu/ptiskus/reactions/Index.htm 1. Write the word equation 2. Write the balanced formula equation ■ Solid iron (III) sulfide reacts with gaseous hydrogen chloride to form iron (III) chloride and hydrogen sulfide gas. 1. Write the word equation 2. Write the balanced formula equation ■ Nitric acid reacts with solid sodium carbonate to form liquid water and carbon dioxide gas and sodium nitrate.