* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download File - Mr Weng`s IB Chemistry
Survey
Document related concepts
Cracking (chemistry) wikipedia , lookup
Homoaromaticity wikipedia , lookup
Marcus theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Aromaticity wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Asymmetric induction wikipedia , lookup
Petasis reaction wikipedia , lookup
Aromatization wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Hydroformylation wikipedia , lookup
Transcript
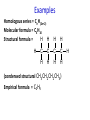
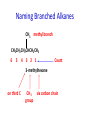
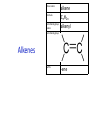
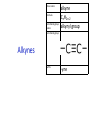
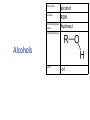
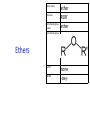
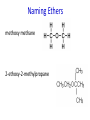
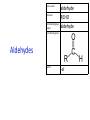
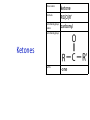
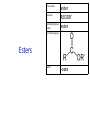
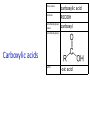
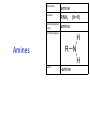
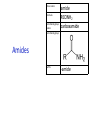
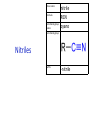
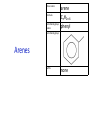
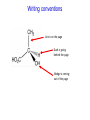
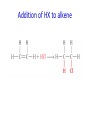
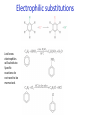
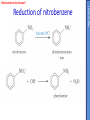
IB CHEMISTRY Topic 10 Organic chemistry Higher level 10.1 Fundamentals of organic chemistry OBJECTIVES • A homologous series is a series of compounds of the same family, with the same general formula, which differ from each other by a common structural unit. • Structural formulas can be represented in full and condensed format. • Structural isomers are compounds with the same molecular formula but different arrangements of atoms. • Functional groups are the reactive parts of molecules. • Saturated compounds contain single bonds only and unsaturated compounds contain double or triple bonds. • Benzene is an aromatic, unsaturated hydrocarbon. • Explanation of the trends in boiling points of members of a homologous • Identification of different classes: alkanes, alkenes, alkynes, halogenoalkanes, alcohols, ethers, aldehydes, ketones, esters, carboxylic acids, amines, amides, nitriles and arenes. • Identification of typical functional groups in molecules eg phenyl, hydroxyl, carbonyl, carboxyl, carboxamide, aldehyde, ester, ether, amine, nitrile, alkyl, alkenyl and alkynyl. • Construction of 3-D models (real or virtual) of organic molecules. • Application of IUPAC rules in the nomenclature of straight-chain and branched-chain isomers. • Identification of primary, secondary and tertiary carbon atoms in halogenoalkanes and alcohols and primary, secondary and tertiary nitrogen atoms in amines. • Discussion of the structure of benzene using physical and chemical evidence. Father of Organic Chemistry Friedrich Wöhler Terminology Hydrocarbon compounds that contain mostly hydrogen and carbon Homologous series compounds with the same general formula Molecular formula shows the number of atoms only Structural formula shows how the atoms are arranged Empirical formula shows lowest whole number ratio of atoms Examples Homologous series = CnH(2n+2) Molecular formula = C4H10 H H Structural formula = H H H C C C C H H H H (condensed structural CH3CH2CH2CH3) Empirical formula = C2H5 H Homologous series The general trend for all molecules in a homologous series is an increase in the boiling point. This is because of the effect of London Dispersion Forces and the molecular weights of the lengthening carbon chains. Structural Isomers Structural isomer same molecular formula but different structure H H H H C C C H H H H C H H BRANCHED CHAIN CH3CH2(CH2)CH3 H H H H H C C C C H H H H STRAIGHT CHAIN CH3CH2CH2CH3 H Functional groups Atoms or groups of atoms attached to a hydrocarbon They are usually the reactive groups on a stable carbon chain so form the important part of the molecule Examples: • alcohols • aldehydes • ketones • carboxylic acids • halides (halogenoalkanes) Class name Formula Functional group name Functional group alkane CnH2n+2 alkyl none Alkanes Suffix -ane Alkanes Name Number of carbons Condensed Structural Formula Methane 1 CH4 Ethane 2 CH3CH3 Propane 3 CH3CH2CH3 Butane 4 CH3CH2CH2CH3 Pentane Hexane 5 6 CH3CH2CH2CH2CH3 CH3CH2CH2CH2CH2CH3 Alkyl Groups Branches on carbon chains H C H H H H C C H H H CH3 methyl CH2CH3 ethyl IUPAC Naming Summary 1. Count the C’s in the longest chain. 2. Name each attached group. 3 Count the longest carbon chain to give the first attached group the smallest number. 4. Name and locate each group. Naming Branched Alkanes CH3 methyl branch CH3CH2CH2CHCH2CH3 6 5 4 3 2 1 Count 3-methylhexane on third C CH3 group six carbon chain Class name Formula Functional group name alkene CnH2n alkenyl Functional group Alkenes Suffix -ene Alkenes Name Number of carbons Condensed Structural Formula Ethene 2 CH2CH2 Propene 3 CH2CHCH3 Butene 4 CH2CHCH2CH3 Pentene Hexene 5 6 CH2CHCH2CH2CH3 CH2CHCH2CH2CH2CH3 Naming Branched Alkanes • select the longest chain of C atoms containing the double bond and number the chain from this end • place the ending ENE on the basic name • use a number to indicate the lower number carbon of the C=C • as in alkanes, prefix with substituents • side chain positions are based on the number allocated to the first C of the C=C e.g. CH3 - CH = CH - CH2 - CH(CH3) - CH3 5-methylhex-2-ene Structural isomerism in alkenes Different structures are possible due to... Different positions for the double bond pent-1-ene pent-2-ene Branching 3-methybut-1-ene Class name Formula Functional group name alkyne CnH2n-2 alkynyl group Functional group Alkynes Suffix -yne Alkynes Name Number of carbons Condensed Structural Formula Ethyne 2 CHCH Propyne 3 CHCCH3 Butyne 4 CHCCH2CH3 Pentyne Hexyne 5 6 CHCCH2CH2CH3 CHCCH2CH2CH2CH3 Saturated vs unsaturated Saturated hydrocarbons are hydrocarbons that contain no double or triple bonds (alkanes). Unsaturated hydrocarbons are alkenes and alkynes. Class name Formula Functional group name halogenoalkane CnH2n+1X X = F, Cl, Br, I Functional group Halogenoalkanes Suffix Prefix none flouro-, chloro-, bromo-, iodo- Halides and naming Different positions for the halogen and branching of the carbon chain 1-chlorobutane 2-chloro-2-methylpropane 2-chlorobutane 1-chloro-2-methylpropane Halides The number of carbons that are joined to the carbon with the halogen group determine if it is a 1⁰, 2⁰, or 3⁰ halide. PRIMARY 1° SECONDARY 2° If you remove this carbon it is still primary TERTIARY 3° Class name Formula Functional group name alcohol ROH hydroxyl Functional group Alcohols Suffix -ol Naming Alcohols Full structure formula Skeletal Name Formula Ethanol Propan-1-ol Propan-2-ol Full structure formula Skeletal Name Formula Butan-1-ol Pentan-1-ol Butan-2-ol Pentan-3-ol Alcohols • The number of carbons that are joined to the carbon with the alcohol group determine if it is a 1⁰, 2⁰, or 3⁰ alcohol. Class name Formula Functional group name ether ROR’ ether Functional group Ethers Suffix Midfix none -oxy- Naming Ethers methoxy methane 2-ethoxy-2-methylpropane Class name Formula Functional group name aldehyde RCHO aldehyde Functional group Aldehydes Suffix -al Naming Aldehydes CARBONYL COMPOUNDS - NOMENCLATURE CH3CHO ethanal CH3CH2CHO propanal CH3CH2CH2CHO butanal CH3CH2CH2CH2CHO pentanal Class name Formula Functional group name ketone RC(O)R’ carbonyl Functional group Ketones Suffix -one Naming Ketones CH3COCH3 propanone CH3CH2COCH3 butanone CH3COCH2CH2CH3 pentan-2-one CH3CH2COCH2CH3 pentan-3-one Class name Formula Functional group name ester RCOOR’ ester Functional group Esters Suffix -oate Naming Esters •The first part is from the alcohol group (of a carboxylic acid), the second part is from the acid (of a carboxylic acid). •Add oate e.g. methyl ethanoate CH3COOCH3 METHYL ETHANOATE ETHYL METHANOATE Class name Formula Functional group name carboxylic acid RCOOH carboxyl Functional group Carboxylic acids Suffix -oic acid Carboxylic acids Carboxylic acids form a homologous series HCOOH CH3COOH C2H5COOH With more carbon atoms, there can be structural isomers C3H7COOH (CH3)2CHCOOH NAMING CARBOXYLIC ACIDS Naming Carboxylic acids • select the longest chain of C atoms containing the COOH group; • remove the e and add oic acid after the basic name • number the chain starting from the end nearer the COOH group • as in alkanes, prefix with alkyl substituents • side chain positions are based on the C in COOH being 1 e.g. CH3 - CH(CH3) - CH2 - CH2 - COOH 4-methylpentanoic acid Class name Formula Functional group name amine RNH2 (H=R) amino Functional group Amines Suffix -amine Naming Amines Nomenclature Named after the groups surrounding the nitrogen + amine C2H5NH2 ethylamine (CH3)2NH dimethylamine (CH3)3N trimethylamine C6H5NH2 phenylamine (aniline) Class name Formula Functional group name amide RCONH2 carboxamide Functional group Amides Suffix -amide Naming Amides • Solids named from the corresponding acid • Remove oic acid, add amide CH3CONH2 ethanamide (acetamide) C2H5CONHC6H5 N - phenyl propanamide - the N tells you the substituent is on the nitrogen Class name Formula Functional group name nitrile RCN cyano Functional group Nitriles Suffix -nitrile Naming Nitriles CH3CH2CH2C≡N butanenitrile ethanenitrile Class name Formula Functional group name arene CnH2n-6 phenyl Functional group Arenes Suffix none Benzene or or Due to the resonance energy or stabilization energy of benzene, it is reluctant to undergo addition reactions, but will undergo substitution reactions. Delocalization minimizes the repulsion between electrons. Physical evidence for benzene stability Naming Arenes Arenes are compounds with a benzene ring. Aliphatics on the other hand are compounds without a benzene ring such as alkanes and alkenes. Bromobenzene 1,2-diphenylethyne Physical Characteristics of the functional groups • Explain volatility between functional groups (Hbonding and Van der Waals) • Explain solubility Effects of lengthening chain and branching 10.2 Functional group chemistry OBJECTIVES Alkanes: • Alkanes have low reactivity and undergo free-radical substitution reactions. • Writing equations for the complete and incomplete combustion of hydrocarbons. • Explanation of the reaction of methane and ethane with halogens in terms of a free-radical substitution mechanism involving photochemical homolytic fission. Alkenes: • Alkenes are more reactive than alkanes and undergo addition reactions. Bromine water can be used to distinguish between alkenes and alkanes. • Writing equations for the reactions of alkenes with hydrogen and halogens and of symmetrical alkenes with hydrogen halides and water. • Outline of the addition polymerization of alkenes. • Relationship between the structure of the monomer to the polymer and repeating unit. Benzene: • Benzene does not readily undergo addition reactions but does undergo electrophilic substitution reactions Alcohols: • Alcohols undergo nucleophilic substitution reactions with acids (also called esterification or condensation) and some undergo oxidation reactions. • Writing equations for the complete combustion of alcohols. • Writing equations for the oxidation reactions of primary and secondary alcohols (using acidified potassium dichromate(VI) or potassium manganate(VII) as oxidizing agents). Explanation of distillation and reflux in the isolation of the aldehyde and carboxylic acid products. • Writing the equation for the condensation reaction of an alcohol with a carboxylic acid, in the presence of a catalyst (eg concentrated sulfuric acid) to form an ester. Halogenoalkanes: • Halogenoalkanes are more reactive than alkanes. They can undergo (nucleophilic) substitution reactions. A nucleophile is an electron-rich species containing a lone pair that it donates to an electron-deficient carbon. • Writing the equation for the substitution reactions of halogenoalkanes with aqueous sodium hydroxide. Polymers: • Addition polymers consist of a wide range of monomers and form the basis of the plastics industry. . Writing conventions Double barbed arrow to indicate movement of an electron pair. Single barbed arrow to indicate the movement of a single electron. Single large dot means a radical. Not a single electron but an incomplete valence shell. Square brackets means the charge is distributed over the complex. Dashed lines to indicated electrons shared over more than one bond. [Co(NO2)6]3- Writing conventions Line is on the page Dash is going behind the page Wedge is coming out of the page Alkane reactions Combustion Photochemical substitution (halogenation) Alkanes – chemical properties Carbon in general: CATENATION is the ability to form bonds between atoms of the same element. Carbon forms chains and rings, with single, double and triple covalent bonds, because it is able to FORM STRONG COVALENT BONDS WITH OTHER CARBON ATOMS In particular alkanes: - fairly unreactive; (old family name, paraffin, meant little reactivity) - have relatively strong, almost NON-POLAR, SINGLE covalent bonds -they have no real sites that will encourage substances to attack them Alkanes – chemical properties Carbon forms a vast number of carbon compounds because of the strength of the C-C covalent bond. Other Group IV elements can do it but their chemistry is limited due to the weaker bond strength. BOND ATOMIC RADIUS BOND ENTHALPY C-C 0.077 nm +348 kJmol-1 Si-Si 0.117 nm +176 kJmol-1 The larger the atoms, the weaker the bond. Shielding due to filled inner orbitals and greater distance from the nucleus means that the shared electron pair is held less strongly. Alkanes – combustion reactions 2O2(g) CO2(g) + complete combustion CH4(g) + incomplete combustion CH4(g) + 1½O2(g) CO(g) 2H2O(l) + 2H2O(l) Homolytic and heterolytic fission There are 3 ways to split the shared electron pair in an unsymmetrical covalent bond. UNEQUAL SPLITTING produces IONS known as HETEROLYSIS or HETEROLYTIC FISSION EQUAL SPLITTING produces RADICALS known as HOMOLYSIS or HOMOLYTIC FISSION • • • • If several bonds are present the weakest bond is usually broken first Energy to break bonds can come from a variety of energy sources - heat / light In the reaction between methane and chlorine either can be used, however... In the laboratory a source of UV light (or sunlight) is favoured. Alkanes – free radical mechanism (substitution reactions) Homolytic fission substitution reaction where electrons are shared when a bond is broken Free radical atom or molecule with one free unpaired electron Mechanism to be known Methane + Cl2 Cl2 ——> 2Cl• Initiation RADICALS CREATED Fish hook arrows represent single electrons During initiation, the WEAKEST BOND IS BROKEN as it requires less energy. There are three possible bonds in a mixture of alkanes and chlorine. 412 348 242 Average bond enthalpy kJ mol-1 The Cl-Cl bond is broken in preference to the others as it is the weakest and requires requires less energy to separate the atoms. Mechanism to be known Methane + Cl2 1. Initiation 2. Propagation Must start and end with a radical. 3. Termination Mechanism to be known Methane + Br2 Homolytic fission making 2 free radicals Bromomethane CH4 + Br2 CH3Br + HBr UV (Hydrogen bromide is a colourless gas) ie. Brown to clear Alkene reactions Addition Polymerization Properties of alkenes Carbon atoms are sp2 hybridized at 120⁰ with an outer π bond that is the site of reactivity allowing range of addition reactions. As the bonds in double carbon bonds are not as stable as single bonds (C-C 348 kJ mol-1, C=C 612 kJ mol-1 ie. not 2x348) they are energetically favourable to be converted to single bonds. Also they have a high electron density making their activation energy low. Addition of hydrogen to alkene Addition of HX to alkene Bromine water • Alkenes react with Bromine water. • The bromine water changes from brown to clear. • Tube A must contain an alkene Alkene Alkane Bromine Water Addition of halogen to alkene Addition of water to alkene Polymerization Alkenes can be added together to make long polymer molecules. Here ethene makes the plastic polyethene. Alkene summary Alcohol reactions Combustion Oxidation Esterification Complete combustion of alcohols • This is an extreme form of oxidation • Like all organic compounds they give CO2 & H2O – in excess O2(g) • They burn more cleanly than their equivalent alkanes – O in the compound is available for combustion products so less CO made in limited O2 conditions (incomplete combustion). Alcohols with longer chains have greater molar enthalpies, but may not be able to burn cleanly. C2H5OH(l) + 3O2(g) 2CO2(g) + 3H2O(l) NB: Watch to count the O in the alcohol when balancing! Oxidation of alcohols Oxidation of primary alcohols Primary alcohols are easily oxidised to aldehydes e.g. CH3CH2OH(l) + [O] ethanol ——> KMnO4 or K2Cr2O7 CH3CHO(l) + H2O(l) ethanal it is essential to distil off the aldehyde before it gets oxidised to the acid CH3CHO(l) + [O] ——> CH3COOH(l) ethanal KMnO4 or K2Cr2O7 ethanoic acid Practical details • • • • • the alcohol is dripped into a warm solution of acidified KMnO4 or K2Cr2O7 K2Cr2O7 is reduced from orange Cr(VI) to green Cr(III) aldehydes have low boiling points - no hydrogen bonding - they distil off immediately if it didn’t distil off it would be oxidised to the equivalent carboxylic acid to oxidise an alcohol straight to the acid, reflux the mixture compound formula intermolecular bonding boiling point ETHANOL C2H5OH HYDROGEN BONDING 78°C ETHANAL CH3CHO DIPOLE-DIPOLE 23°C ETHANOIC ACID CH3COOH HYDROGEN BONDING 118°C Oxidation of primary alcohols Controlling the products e.g. CH3CH2OH(l) + [O] ——> CH3CHO(l) + H2O(l) then CH3CHO(l) + [O] ——> CH3COOH(l) KMnO4 or K2Cr2O7 OXIDATION TO ALDEHYDES DISTILLATION OXIDATION TO CARBOXYLIC ACIDS REFLUX Aldehyde has a lower boiling point so distils off before being oxidised further Aldehyde condenses back into the mixture and gets oxidised to the acid Oxidation of secondary alcohols Secondary alcohols are easily oxidised to ketones e.g. CH3CHOHCH3(l) + [O] ——> propan-2-ol KMnO4 or K2Cr2O7 CH3COCH3(l) + H2O(l) propanone The alcohol is refluxed with acidified K2Cr2O7. However, on prolonged treatment with a powerful oxidising agent they can be further oxidised to a mixture of acids with fewer carbon atoms than the original alcohol. Oxidation of tertiary alcohols Tertiary alcohols are resistant to normal oxidation Condensation - Esterification Halogenoalkene reactions Nucleophic substitution Terminology Nucleophile Reactants with a non-bonding electron pair that are attracted to a positive carbon (a form of electrophile – loves negative) Heterolytic fission Formation of a carbocation and a negative ion, due to carbon losing it’s shared electron As the carbon-halogen bond means carbon is slightly positive, halogenoalkanes are reactive and undergo nucleophilic substitution reactions from nucleophile attack. Halogenoalkane substitution reactions General formula: Eg. with NaOH CH3CH(Cl)CH2CH3 + NaOH CH3CH=CHCH3 + NaCl + H2O Conditions: Heat (boil) Benzene reactions Electrophilic substitution Electrophilic substitutions Just know electrophiles will substitute. Specific reactions do not need to be memorized. OBJECTIVES Nucleophilic Substitution Reactions: • SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular substitution reaction. SN1involves a carbocation intermediate. SN2 involves a concerted reaction with a transition state. • For tertiary halogenoalkanes the predominant mechanism is SN1and for primary halogenoalkanes it is SN2. Both mechanisms occur for secondary halogenoalkanes. • The rate determining step (slow step) in an SN1reaction depends only on the concentration of the halogenoalkane, rate = k[halogenoalkane]. For SN2, rate = k[halogenoalkane][nucleophile]. SN2 is stereospecific with an inversion of configuration at the carbon. •SN2 reactions are best conducted using aprotic, non-polar solvents and SN1reactions are best conducted using protic, polar solvents. • Explanation of why hydroxide is a better nucleophile than water. • Deduction of the mechanism of the nucleophilic substitution reactions of halogenoalkanes with aqueous sodium hydroxide in terms of SN1and SN2 mechanisms. Explanation of how the rate depends on the identity of the halogen (ie the leaving group), whether the halogenoalkane is primary, secondary or tertiary and the choice of solvent. • Outline of the difference between protic and aprotic solvents Electrophilic Addition Reactions: • An electrophile is an electron-deficient species that can accept electron pairs from a nucleophile. Electrophiles are Lewis acids. • Markovnikov’s rule can be applied to predict the major product in electrophilic addition reactions of unsymmetrical alkenes with hydrogen halides and interhalogens. The formation of the major product can be explained in terms of the relative stability of possible carbocations in the reaction mechanism. •Deduction of the mechanism of the electrophilic addition reactions of alkenes with halogens/interhalogens and hydrogen halides. Electrophilic Substitution Reactions: • Benzene is the simplest aromatic hydrocarbon compound (or arene) and has a delocalized structure of π bonds around its ring. Each carbon to carbon bond has a bond order of 1.5. Benzene is susceptible to attack by electrophiles. • Deduction of the mechanism of the nitration (electrophilic substitution) reaction of benzene (using a mixture of concentrated nitric acid and sulfuric acid). Reduction Reactions: • Carboxylic acids can be reduced to primary alcohols (via the aldehyde). Ketones can be reduced to secondary alcohols. Typical reducing agents are lithium aluminium hydride (used to reduce carboxylic acids) and sodium borohydride. • Writing reduction reactions of carbonyl containing compounds: aldehydes and ketones to primary and secondary alcohols and carboxylic acids to aldehydes, using suitable reducing agents. • Conversion of nitrobenzene to phenylamine via a two-stage reaction. Higher level 20.1 Types of organic reactions Higher level Nucleophilic Substitution Reactions SN1 and SN2 Polar – have dipole moments due to different electronegativities Non-polar – similar electronegativities Protic – polar solvents with O-H or N-H bonds allowing hydrogen bonding and a source of protons eg. water, ethanol Aprotic – a polar or non-polar solvent that do not have O-H or N-H bonds nor provide a source of protons eg. acetone (propanone) (polar), ethanenitrile (polar), benzene (non-polar), hexane (non-polar) SUMMARY: 3 main types of solvents, non-polar, polar protic, polar aprotic, no such thing as a non-polar protic Higher level Solvents SN2 reactions involve heterolytic fission and nucleophilic substitution with mainly primary halogenoalkanes. An unstable transition state is created meaning the reaction is bimolecular requiring two molecules: rate = k[halogenoalkane][nucleophile] Polar aprotic solvents are preferred which are those unable to form hydrogen bonds, eg. propanone, ethyl ethanoate. Otherwise the solvent would bind to the nucleophile inhibiting its action. Higher level SN2 reaction mechanism SN2 reaction mechanism Inversion at 180⁰ must be shown. Higher level Mechanism to be known SN1 reactions involve heterolytic fission and nucleophilic substitution with mainly tertiary halogenoalkanes. Due to steric hindrance the halogen must be heterolytically removed to create a carbocation before the nucleophile can attached. This creates a more stable carbocation intermediate so the reaction is unimolecular: rate = k[halogenoalkane] Polar protic solvents are preferred which are able to form hydrogen bonds which stabilize the carbocation by ion-dipole interactions eg. water, alcohol Higher level SN1 reaction mechanism The positive induction is the stabilization of a carbocation because the other alkyl groups can unevenly share their electrons with the positive centered carbon: Higher level Positive induction SN1 reaction mechanism Higher level Mechanism to be known As halogen-carbon bonds become less polar you would expect nucleophilic attack to be less and so rate to decrease down the group: However we must also consider how much energy it takes to break bonds: And this is the deciding factor meaning rate increases down the group: Higher level Effect of leaving group In general SN1 tertiary reaction rates are faster than SN2 primary due to the stability of the formation of carbocations: Higher level Effect of mechanism Higher level Electrophilic Addition Reactions Alkenes Ethene and bromine Higher level Mechanism to be known Ethene and HBr Higher level Mechanism to be known Markovnikov’s rule: the hydrogen will attach to the carbon that is already bonded to the greater number of hydrogens. In the following situation: b. Will be more stable due to positive induction: Hence reaction b. prevails. Higher level Asymmetric alkenes Higher level Propene and HBr Higher level Electrophilic Substitution Reactions Benzene Nitration of benzene Firstly sulfuric acid is stronger so protonates the nitric acid: With heat, the electrophilic substitution by the nitronium ion causes a momentary loss of symmetry of the electron structure of benzene. Higher level Mechanism to be known Higher level Reduction Reactions Alcohols Nitrobenzene The oxidation of alcohols can be reversed by reduction as follows: [+H-] [+H-] Needs lithium aluminium hydride (LiAlH4) in dry ether. [+H-] Needs heat with sodium borohydride (NaBH4). Higher level Reduction of carbonyl compounds Reduction of nitrobenzene Higher level Mechanism to be known? OBJECTIVES • The synthesis of an organic compound stems from a readily available starting material via a series of discrete steps. Functional group interconversions are the basis of such synthetic routes. • Retro-synthesis of organic compounds. • Deduction of multi-step synthetic routes given starting reagents and the product(s). Higher level 20.2 Synthetic routes A synthetic route is a series of discrete chemical steps to change a reactant to a desired product. Efficient design has been the result of retrosynthetic analysis where you think of the target molecule and work backwards through precursors to the starting material. Higher level Synthetic routes aldehyde phenylamine (aniline) Reflux Oxidation M Reduction LiAlH4 dry ether Cr2O72- H+ Cr2O72- H+ Reduction 1⁰ Oxidation LiAlH4 dry ether carboxylic acid NaOH 2⁰ Oxidation alcohol ketone Reduction Condensation phenylammonium ion M NaBH4 halogenoalkane M 1⁰ SN2, 3⁰ SN1 H2O/H2SO4 Substitution Addition M Sn conc. HCl dihalogenoalkane Cl2 UV HCl Free radical Addition nitrobenzene M Cl2 M Addition Homolytic fission alkane M ester NaOH Conc. HNO3O/H2SO4 Ni H2 Addition Addition alkene polymerization polymer benzene Higher level Cr2O72- H+ Higher level Problem 1: Produce ethanol from ethane. OBJECTIVES • Stereoisomers are subdivided into two classes—conformational isomers, which interconvert by rotation about a σ bond and configurational isomers that interconvert only by breaking and reforming a bond. Configurational isomers are further subdivided into cis-trans and E/Z isomers and optical isomers. • Cis-trans isomers can occur in alkenes or cycloalkanes (or heteroanalogues) and differ in the positions of atoms (or groups) relative to a reference plane. According to IUPAC, E/Z isomers refer to alkenes of the form R1R2C=CR3R4 (R1 ≠ R2, R3 ≠ R4) where neither R1 nor R2 need be different from R3 or R4. • A chiral carbon is a carbon joined to four different atoms or groups. • An optically active compound can rotate the plane of polarized light as it passes through a solution of the compound. Optical isomers are enantiomers. Enantiomers are non-superimposeable mirror images of each other. Diastereomers are not mirror images of each other. • A racemic mixture (or racemate) is a mixture of two enantiomers in equal amounts and is optically inactive. • Construction of 3-D models (real or virtual) of a wide range of stereoisomers. • Explanation of stereoisomerism in non-cyclic alkenes and C3 and C4 cycloalkanes. • Comparison between the physical and chemical properties of enantiomers. • Description and explanation of optical isomers in simple organic molecules. • Distinction between optical isomers using a polarimeter. Higher level 20.3 Stereoisomerism S C Higher level Isomers Cis isomers are those with the same groups on the same side of the double bond or cyclical compounds. Trans isomers have the groups on the opposite sides of the double bond. Higher level Cis-trans isomers Higher level Example: Higher level E/Z isomers For isomers with more than 2 different groups, the group with the highest priority on the left hand side is determined and then the group with the highest priority on the right hand side is determined. E isomers have these two groups opposite. (entgegen in German) Z isomers have these two groups on the same side. (above) (zusammen in German) Z is S in a mirror Priority rules: 1. The atom with the highest atomic number has the highest priority. 2. If the atom is the same, apply this rule to the next bonded atom in the chain. Higher level Example: Higher level Different compounds - different properties. Optical isomers are those which contain a chiral carbon, that is, it contains four different groups. These compounds are nonsuperimposable on each other and exist in pairs called enantiomers eg. amino acids. Diastereomers contain two or more chiral carbons and are not mirror images. Higher level Optical isomers • isomers differ in their reaction to plane-polarised light • plane polarised light vibrates in one direction only • one isomer rotates light to the right, the other to the left (degree of angle equal) • rotation of light is measured using a polarimeter • rotation is measured by observing the polarised light coming out towards the observer • If the light appears to have turned to the right DEXTROROTATORY d or + form turned to the left LAEVOROTATORY l or - form A racemate or racemic mixture is a 50-50 mixture of the two enantiomers (dl) or (±). The opposite optical effects of each isomer cancel each other out. Higher level Optical activity Higher level Polarimeter A B C D E F A B C D E F Light source produces light vibrating in all directions Polarising filter only allows through light vibrating in one direction Plane polarised light passes through sample If substance is optically active it rotates the plane polarised light Analysing filter is turned so that light reaches a maximum Direction of rotation is measured coming towards the observer If the light appears to have: turned to the right DEXTROROTATORY turned to the left LAEVOROTATORY • The formation of racemic mixtures is more likely in a laboratory reaction than in a chemical process occurring naturally in the body. • If a compound can exist in more than one form, only one of the optical isomers is usually effective. • The separation of isomers will make manufacture more expensive. • A drug made up of both isomers will require a larger dose and may cause problems if the other isomer is ‘poisonous’ like thalidomide, which is a teratogen – causes birth defects. Higher level Optical isomers – Other points (which is different to natural selection!) Only L-forms of amino acids are used in cells. Aren’t proteins a violation of entropy and enthalpy anyway? If there are no proteins, how did the DNA reproduce? Which came first the chicken or the egg? Higher level TOK - problems for evolution?