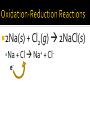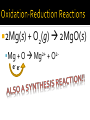* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Oxidation Reduction PowerPoint
Chemical equilibrium wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Nuclear fusion wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Catalytic reforming wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Water splitting wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Acid–base reaction wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Process chemistry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Rate equation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Inorganic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Asymmetric induction wikipedia , lookup
Organic chemistry wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Electrochemistry wikipedia , lookup
Marcus theory wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Photosynthesis wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Ene reaction wikipedia , lookup
Stoichiometry wikipedia , lookup
Click chemistry wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical reaction wikipedia , lookup
Chapter 6: Chemical Reactions Identify redox reactions. Identify and write equations for combustion reactions. A reaction can be classified as a redox reaction if it meets any one of these requirements. A substance reacts with elemental oxygen (combustion). A metal reacts with a nonmetal. During single displacement reactions. More generally, one substance transfers electrons to another substance. 2Na(s) + Cl2(g) 2NaCl(s) Na + Cl Na+ + Cle- Combustion reactions are a type of redox reaction. Combustion reactions are characterized by the reaction of a substance with O2 to form one or more oxygen-containing compounds, often including water. Combustion reactions are exothermic (they emit heat). Compounds containing carbon and hydrogen always form carbon dioxide and water upon combustion. CH4(g) + O2(g) 2Mg(s) + O2(g) 2MgO(s) Mg + O e- e- 2+ Mg 2+O 2Al(s) + Fe2O3(s) 2Fe(s) + Al2O3(s) Al + Fe3+ Fe + Al3+ e- -ee For each reaction, show how electrons are gained and lost: 2Na (s) + Br2(l) 2NaBr (s) 2Ca (s) + O2(g) 2CaO (s) Predict the products for the following combustion reactions: C6H14(l) + O2(g) Cu(s) + O2(g) Classify each of the following reactions in as many ways possible. 2K (s) + Cl2(g) 2KCl (s) 2Mg (s) + O2(g) 2MgO (s) Classify each of the following reactions in as many ways possible. Fe2O3(s) + 2Al(s) Al2O3(s) + 2Fe(s) HNO3(aq) + NaOH(aq) → H2O(l) + NaNO3 (aq) Ba(NO3)2(aq) + (NH4)2SO4(aq) HCl(aq) + KOH(aq) Na(s) + Cl2(g) FeCl3(aq) + NaOH(aq) Zn(s) + HNO3(aq) C6H14(l) + O2(g) CuSO4(aq) + Na2CO3(aq) HClO4(aq) + NaOH(aq) NI3(s) ZnCl2(aq) + Mg(s) H2SO4(aq) + Ba(OH)2(aq) KNO3(aq) + BaCl2(aq)