* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download introduction into Analytical Chemistry
Drug discovery wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Chemical equilibrium wikipedia , lookup
History of electrochemistry wikipedia , lookup
Inorganic chemistry wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Safety data sheet wikipedia , lookup
Fine chemical wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
History of chemistry wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Acid strength wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Institute of Chemistry Ceylon wikipedia , lookup
Crystallization wikipedia , lookup
Acid–base reaction wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Chemical industry wikipedia , lookup
Computational chemistry wikipedia , lookup
Green chemistry wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Heap leaching wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
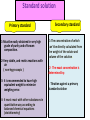
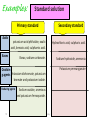
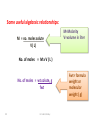
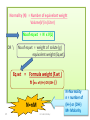
Pharmaceutical Analytical Chemistry / PHC 213 Contact Information Building : 2 2nd floor Office : 48 Contact me on Email : [email protected] . [email protected] Twitter : @Halah_almutairi Moblie No : 0534790301 Analytical Chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials Analytical chemistry answer two important questions How much is it ?? What is it ?? (quantitative analysis) (Qualitative analysis) determines the amount gives an indication of the (concentration) of one or identity of the more of the components chemical species in the in the sample sample Classification of Analytical Methods : Gravimetric method Volumetric method Instrumental methods Gravimetric method : Gravimetric methods of analysis are based on the measurement of mass. Volumetric method (Titration(: involves the addition of a reactant to a solution being analyzed until some equivalence point is reached Types of Titration: Acid _ base titration Precipitimetric titration Complexmetric titration Redox(oxidation-reduction) titration Instrumental methods : Spectrophotometry Chromatography Application of Analytical Chemistry : Analytical chemistry play an important role in nearly all aspect of chemistry Medicine Industry Environmental Food and Agriculture Quality control Application of Analytical Chemistry : In medicine, analytical chemistry is the basis for clinical laboratory tests which help the physicians diagnose disease In industry, analytical chemistry provides the means of testing the raw materials for assuring the quality of finished products whose chemical composition is critical (eg. Drugs ) The nutritional value of food determined by chemical analysis for major component such as protein and carbohydrate and trace components such as vitamins and minirals Definition of some terms : Sample: is a material that we wish to analyze Analyte: is the substance or element in the sample whose presence or concentration we wish to determine titrant: is a solution of known concentration which is added (titrated) to another solution to determine the concentration of second chemical species Titration: is a process which is performed by slow addition of standard solution "titrant" from a burette to a solution of the analyte until the reaction between the two is complete. Standard Solution: - is a solution of known concentration - prepared by dissolving a known amount of the substance (primary standard substance)in a known volume of liquid - They provide a reference to determine unknown concentrations -Two types,, primary and secondary standard solution Standard solution Secondary standard Primary standard 1-Must be easily obtained in very high grade of purity and of known composition. 2-Very stable, and resists reactions with air ( non-hygroscopic ) 3- It is recommended to have high equivalent weight to minimize weighing error. 1-The concentration of which can’t be directly calculated from the weight of the solute and volume of the solution. 2- The exact concentration is determined by: - Titration against a primary standard solution 4- It must react with other substances in quantitative way according to balanced chemical equations (stoichiometry) 17 Dr. Hadir Shalaby Examples: Standard solution Primary standard Acids Secondary standard potassium acid phthalate, oxalic acid, benzoic acid, sulphamic acid. Hydrochloric acid, sulphuric acid. Borax, sodium carbonate. Sodium hydroxide, ammonia Oxidizin g agents Potassium dichromate, potassium bromate and potassium iodate. Potassium permanganate Bases Reducing agents Sodium oxalate, arsenious oxide, and potassium ferrocyanide. 18 Dr. Hadir Shalaby Equivalence point: The point in a titration when the amount of added standard reagent is exactly equal to ( is chemically equivalent to) the amount of the analyte. End point: The point in a titration when a physical change occurs that is associated with the condition of chemical equivalence. Volume difference between the equivalence point and the end point should be small . This difference in volume is the titration error 19 Detection of the end point Indicator : a substance that indicate the presence, absence, or concentration of another substance ,,often used in a titration to indicate the point at which the reaction is complete by means of a characteristic change, especially in color such as: litmus paper in acid media in base media Methods of expressing concentration of standard solutions I-Molarity Molar solution: It is a solution of the substance containing one mole (gram molecular weight) of the substance per one liter of solution. Molarity (M) : It is the number of moles (gram molecular weight )of solute per one liter of solution. 21 Dr. Hadir Shalaby Some useful algebraic relationships: M = no. moles solute V( L) M=Molarity V=volume in liter No. of moles = M x V ( L ) No. of moles = wt.solute, g fwt 22 Dr. Hadir Shalaby Fwt= formula weight or molecular weight ( g) Examples: Example 1: Calculate the molarity of 17g Na2CO3 in 500ml of solution (fwt=106) Convert 500 ml to liter by M= …… wtg …….. = 17 = 0.320 ÷1000 = 0.5 L fwt × VL 106 × 0.5 Example 2: ( HOME WORK) Calculate the weight in grams of Na2CO3 required to prepare 250ml of 0.15M solution. (fwt=106) 23 Dr. Hadir Shalaby II-Normality II. Normal solution: It is the solution that contains one gram equivalent weight of solute per liter of solution. Normality (N) : It is the number of equivalents (gram equivalent weight) per liter of solution. If the equivalent weight = formula weight so, 24 N=M Dr. Hadir Shalaby Normality (N) = Number of equivelant weight Volume(V) in (Liter) No.of eq.wt = N x V(L) OR \ No.of eq.wt = weight of solute (g) equivalent weight (Eq.wt) Eq.wt = Formula weight (F.wt ) n (no. of (H+) OR (OH-) ) N=nM 25 Dr. Hadir Shalaby N=Normality n = number of (H+) or (OH-) M= Molarity Problem??? Calculate the eq.wt for each of the following: HCl , H2SO4 , H2CO3 , H3PO4 , Ba(OH)2 *In case of HCl or NaOH HCl 1 H+ eqwt = fwt / 1 NaOH 1 OH*In case of Ba(OH)2 Ba(OH)2 2 OHeqwt = fwt / 2 *In case of H3PO4 H3PO4 H2PO4- + H+ eqwt = fwt / 1 H3PO4 HPO4-2 + 2 H+ eqwt = fwt / 2 26 Dr. Hadir Shalaby Examples: 1-How much primary standard potassium acid phthalate, KHC8H4O4, is required to prepare 499.5 ml of 0.10 N solution?(fwt = 204.23) Weight ( g) = ?? Convert 499.5 ml to Liter by ÷1000 = 0.4995 L No of eq.wt = N x V (L) Wt/ eqwt = N x V Wt ( g) = N x V x eqwt = 0.1 x 0.4995 x 204.23 = 10. 20 g KHC8H4O4 27 Dr. Hadir Shalaby TYPES OF WATER USED: • Distilled water: is water that has many of its impurities removed through distillation • Deionized water : is water that has had all minerals removed from it,as cations like sodium, calcium, iron, and copper, and anions such as chloride and sulfate, using an ion exchange process Lab glassware and equipment burette Pipette: Graduated pipettes Volumetric pipettes beaker cylinder funnels Volumetric flask Conical flask: Glass stopper conical flask Conical flask Glass rod Mortar and pestle Hot plate electronic balance hood GENERAL GUIDELINES: 1- When first entering the lab , do not touch any equipment, chemicals, or other materials in the laboratory area until you are instructed to do so 2- Follow all written and verbal instructions carefully. If you do not understand a direction or part of a procedure, ASK YOUR TEACHER BEFORE PROCEEDING WITH THE ACTIVITY 3- Never work alone in the laboratory,No student may work in the laboratory without the presence of the teacher 4- Do not eat food, drink beverages, or chew gum in the laboratory,Do not use laboratory glassware as containers for food or beverages 5- Observe good housekeeping practices , Work areas should be kept clean at all times 7- Labels and equipment instructions must be read carefully before use 8- Keep hands away from face, eyes, mouth, and body while using chemicals , Wash your hands with soap and water after performing all experiments 9-you should Know the locations and operating procedures of all safety equipment CLOTHING: Dress properly during a laboratory activity. Long hair must be tied back, Shoes must completely cover the foot HANDLING CHEMICALS: 1- All chemicals in the laboratory are to be considered dangerous. Avoid handling chemicals with fingers , Do not taste or smell any chemicals 2- Check the label on all chemical bottles twice before removing any of the contents 3- Never return unused chemicals to their original container, and Never remove chemicals or other materials from the laboratory area HANDLING GLASSWARE AND EQUIPMENT: 1- Never handle broken glass with your hands , Place broken glass in the sharps container 2- Examine glassware before each use 3- Never look into a container that is being heated 4- hood (how can we use it in the safe way ) COURSE SYLLABUS: Thank you