* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Regulation of the S100B gene by α1-adrenergic - AJP
Epigenetics of human development wikipedia , lookup
Non-coding RNA wikipedia , lookup
Messenger RNA wikipedia , lookup
Gene therapy wikipedia , lookup
Gene expression programming wikipedia , lookup
Microevolution wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Gene nomenclature wikipedia , lookup
Point mutation wikipedia , lookup
Non-coding DNA wikipedia , lookup
Gene desert wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Epitranscriptome wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Designer baby wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Epigenetics of depression wikipedia , lookup
Transcription factor wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Primary transcript wikipedia , lookup
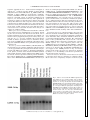
Am J Physiol Heart Circ Physiol 284: H193–H203, 2003. First published September 5, 2002; 10.1152/ajpheart.00161.2002. Regulation of the S100B gene by ␣1-adrenergic stimulation in cardiac myocytes JAMES N. TSOPORIS,1 ALEXANDER MARKS,2 LINDA J. VAN ELDIK,3 DAVID O’HANLON,2 AND THOMAS G. PARKER1 1 Division of Cardiology, Department of Medicine, The Toronto General Hospital Research Institute, University of Toronto, Toronto M5G 2C4; 2Banting and Best Department of Medical Research, University of Toronto, Toronto, Ontario M5G 1L6, Canada; and 3Drug Discovery Program and Department of Cell and Molecular Biology, Northwestern University Medical School, Chicago, Illinois 60611 Tsoporis, James N., Alexander Marks, Linda J. Van Eldik, David O’Hanlon, and Thomas G. Parker. Regulation of the S100B gene by ␣1-adrenergic stimulation in cardiac myocytes. Am J Physiol Heart Circ Physiol 284: H193–H203, 2003. First published September 5, 2002; 10.1152/ajpheart.00161.2002.—We previously reported that S100B, a 20-kDa Ca2⫹-binding homodimer, inhibited the postinfarct myocardial hypertrophic response mediated by ␣1-adrenergic stimulation through the protein kinase C (PKC) signaling pathway. In the present study, we examined whether the same pathway induced the S100B gene, supporting the hypothesis that S100B is a feedback negative regulator of this pathway. We transfected cultured neonatal rat cardiac myocytes with a luciferase reporter gene driven by the maximal human S100B promoter and progressively shorter segments of this promoter sequentially deleted from the 5⬘ end. We identified a basic promoter essential for transcription spanning 162 bp upstream of the transcription initiation site and positive (at ⫺782/⫺162 and ⫺6,689/ ⫺4,463) and negative (at ⫺4,463/⫺782) myocyte-selective regulatory elements. We showed that the basic and maximal S100B promoters were activated specifically by ␣1-adrenergic agonists through the ␣1A-adrenergic receptor, but not by any other trophic hormonal stimuli. The activation of the S100B promoter was mediated through the PKC signaling pathway. Transcription enhancer factor-1 (TEF-1) and related to TEF-1 (RTEF-1) influenced transcription from the maximal, but not the basic, promoter implicating active MCAT elements upstream from the basic promoter. Acting in opposing fashions, TEF-1 transrepressed the S100B promoter and RTEF-1 transactivated the promoter. Our results suggest that ␣1-adrenergic stimulation induces the S100B gene after myocardial infarction through the PKC signaling pathway and that this induction is modulated by TEF-1 and RTEF-1. norepinephrine; TEF-1 MYOCARDIAL INFARCTION, the leading cause of death in the developed world, results in an acute loss of functional myocardium. Among survivors, postinfarct ventricular remodeling is an adaptive response of the heart to this loss in an attempt to preserve cardiac performance Address for reprint requests and other correspondence: T. G. Parker, The Toronto General Hospital, 200 Elizabeth St., EN12-208, Toronto, Ontario M5G 2C4, Canada (E-mail: [email protected]). http://www.ajpheart.org (25). Myocardial hypertrophy is an integral component of this adaptive response. Because adult cardiac myocytes are terminally differentiated and have lost the ability to divide, this increase in mass is due to enlargement of individual myocytes. The hypertrophic response can be reproduced in rat and mouse experimental models and cultured neonatal cardiac myocytes from these species (5, 25, 26). Experimental work in these models has implicated hormonal stimulation, including ␣1-adrenergic agonists, angiotensin (ANG) II, peptide growth factors, and mechanical stretch as instigators of the hypertrophic response (4, 30, 35). Some of these, including ␣1-adrenergic agonists, activate the protein kinase C (PKC) signaling pathway (15, 17). Furthermore, in addition to growth, myocytes have been shown to respond to trophic stimuli in these models by reexpression of several fetal genes, including the embryonic -myosin heavy chain (-MHC), the skeletal isoform of ␣-actin (skACT), and atrial natriuretic factor (4, 15, 17, 35). The relative contributions of the various trophic stimuli that initiate and sustain the hypertrophic response after myocardial infarction have not been defined. Moreover, some form of negative regulation may be involved to limit unchecked hypertrophy. We have previously proposed that S100B, a 20-kDa Ca2⫹-binding homodimer normally expressed in brain astrocytes, was a candidate for an intrinsic negative regulator of the myocardial hypertrophic response on the basis of several experimental observations. First, whereas S100B is normally not expressed in the myocardium, S100B protein was identified immunohistochemically in the peri-infarct region of the human heart after myocardial infarction, and S100B mRNA was detected in the rat heart after coronary artery ligation in an experimental model of myocardial infarction (32, 33). Second, in cotransfection experiments in cultured neonatal rat cardiac myocytes, S100B inhibited the ␣1- The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. 0363-6135/03 $5.00 Copyright © 2003 the American Physiological Society H193 Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 Submitted 27 February 2002; accepted in final form 29 August 2002 H194 ␣1-ADRENERGIC INDUCTION OF S100B IN HEART MATERIALS AND METHODS Human S100B promoter-driven reporter plasmids. A luciferase reporter gene system (Promega; Madison, WI) was used to study the relative ability of different 5⬘ DNA regions of the human S100B gene to promote transcription in transient transfection assays, as previously described (3). In this system, a maximal human S100B promoter and progressively shorter fragments sequentially deleted from the 5⬘ end of the maximal promoter by restriction enzyme digestion were cloned upstream of the luciferase gene into the plasmid pGL3, which lacks eukaryotic enhancer and promoter sequences. Initially, a 9.8-kb HindIII fragment of human genomic DNA (1) spanning exons 1 and 2 (and the intervening intron) of the human S100B gene and 6,689 bp of upstream and 320 bp of downstream DNA sequence, which was numbered in accordance with the human S100B genomic sequence in the National Center for Biotechnology Information database, was subcloned into a BlueScript plasmid (pBS; Stratagene; La Jolla, CA) to generate pBS100 9.1. A HindIII/ BamHI fragment spanning the DNA nucleotide sequence ⫺6,689/⫹698 relative to the transcription initiation site was excised from pBS100 9.1 and designated as the maximal S100B promoter. This fragment includes 6,689 bp of DNA sequence upstream of exon 1, exon 1 (71 bp), and 627 bp of downstream sequence and was subcloned into pGL3 to generate pGBS ⫺6,689/⫹698. The reporter plasmids containing progressively shorter segments of the maximal promoter were generated by subcloning the fragments KpnI/BamHI, PvuII/BamHI, and SmaI/BamHI, excised from pBS100 9.1, into pGL3 to generate pGBS ⫺4,463/⫹698, pGBS ⫺782/ ⫹698, and pGBS ⫺162/⫹698, respectively (3). The plasmid pGBS ⌬⫺162/⫹698 was generated similarly from a fragment AJP-Heart Circ Physiol • VOL created by deleting the region ⫺162/⫹698 from pGBS ⫺4,463/⫹698 (see Fig. 3). Expression plasmids. The expression plasmids were obtained from the following sources: SR␣-⌬PKC- [containing a partially deleted constitutively active PKC- cDNA driven by a sarcoma virus (SV)40 early promoter] was from Dr. P. C. Simpson (14); pXJ40-TEF-1A and pXJ40-RTEF-1 (containing human TEF-1 and RTEF-1 cDNA, respectively, driven by a cytomegalovirus enhancer) were from Dr. A. F. R. Stewart (31); pBK28-c-Fos (containing a human c-Fos cDNA driven by a murine SV long-terminal repeat promoter) and pSV-cJun (containing a human c-Jun cDNA driven by a SV40 early promoter) were from Dr. I. Verma; and Rous sarcoma virus (RSV)-CAT (containing a CAT cDNA driven by a RSV longterminal repeat promoter) was from Stratagene (La Jolla, CA). Plasmids were purified using midi or maxi kits (Qiagen; Valencia, CA). Left coronary artery ligation. Left coronary artery ligation was performed in the rat as previously described and as approved by the animal care committee of the Toronto General Research Institute (24). Briefly, 8-wk-old rats were anesthetized intraperitoneally with 90 mg/kg ketamine and 10 mg/kg xylazine. A left thoracotomy was performed, and a 6-0 silk suture was placed through the myocardium into the anterolateral left ventricular wall. This area corresponds to the course of the left anterior descending artery in the rat. The suture was positioned approximately midway between the apex and base. The suture was then tied and the chest cavity closed. Tissue catecholamine measurements. For tissue norepinephrine (NE) measurements, left ventricular tissue was homogenized in 0.4 N perchloric acid containing 5 mM reduced glutathione and then centrifuged to produce a proteinfree supernatant. Tissue NE was assayed using the Biotrak catecholamines 3H-labeled radioenzymatic assay system (Amersham Pharmacia Biotech; Baie d’Urfe, Quebec, Canada) according to the instructions of the manufacturer. In brief, the assay utilized the enzyme catechol-O-methyltransferase to catalyze the transfer of a 3H labeled methyl group from S-adenosyl-L-[methyl-3H]methionine to NE. The resulting product, [3H]normetanephrine, was isolated with the use of thin-layer chromatography. [3H]normetanephrine was converted by periodate oxidation to [3H]vanillin and extracted. The radioactivity attributable to NE was determined by liquid scintillation counting. Cell transfections. Low-density (⬃150 cells/mm2) noncontractile neonatal cardiac myocytes and fibroblasts were isolated from ventricles of 2-day-old Sprague-Dawley rats and established in culture as previously described (21, 30) and as approved by the animal care committee of the Toronto General Research Institute. Transfection was carried out by calcium phosphate precipitation (14) with the use of specified quantities of the following plasmids: pW9Fc (9 g); pGBS ⫺162/⫹698, pGBS ⌬⫺162/⫹698, pGBS ⫺782/⫹698, pGBS ⫺4,463/⫹698, and pGBS ⫺6,689/⫹698 (5 g); and SR ␣-⌬PKC-, pXJ40-TEF-1A, pXJ40-RTEF-1, pBK28-c-Fos, and pSV-c-Jun (0.1 g), as indicated in the legends. RSVCAT (0.1 g) was included in all plates as an internal control for transfection efficiency. Myocyte cultures were maintained in medium supplemented with 5% fetal bovine serum for 18 h after transfection before transfer to serum-free medium and treatment with 20 M NE, 20 M phenylephrine (PE), 20 M isoproterenol (Iso), 20 ng/ml 3,5,3⬘-triiodothyronine (T3), 100 nM ANG II, the phorbol ester phorbol 12-myristate 13-acetate (PMA; 10 nM), the ␣1A-adrenergic receptor antagonist WB-4101 (2 M), the ␣1B-adrenergic receptor antagonist chloroethylclonidine (CEC) (2 M), the PKC inhibitor stau- 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 adrenergic-mediated induction of -MHC and skACT through the PKC signaling pathway (32). Third, in S100B-overexpressing transgenic mice, S100B protein and mRNA were detected in the heart after ␣1-adrenergic agonist infusion, and the hypertrophic response normally evoked in the heart and cultured myocytes in response to ␣1-adrenergic stimulation in wild-type mice was abrogated (33). The above data suggest that S100B functions as a negative feedback regulator of the ␣1-adrenergic-mediated component of the hypertrophic response after myocardial infarction. A corollary of this suggestion is that the S100B gene is induced after myocardial infarction by the same ␣1-adrenergic pathway. To formally examine this possibility, we used luciferase reporter constructs to study the activation of the human S100B promoter by components of the ␣1-adrenergic signaling pathway in cultured rat myocytes. These components included PKC, and the transcription factors transcription enhancer factor-1 (TEF-1) and related TEF-1 (RTEF-1), which bind to MCAT elements. These elements have previously been reported (14, 16, 17, 19, 31) to be involved in the induction of the fetal genes -MHC and skACT in cultured rat neonatal cardiac myocytes and in the intact rat heart. We report the specific induction of the S100B promoter by ␣1-adrenergic stimulation in rat myocytes through the PKC signaling pathway and the selective modulation of this induction by TEF-1 and RTEF-1 acting through MCAT elements in the S100B promoter. ␣1-ADRENERGIC INDUCTION OF S100B IN HEART thesis of a 210-bp fragment from human RNA (32). The rat S100B primers 5⬘-TTGCCCTCATTGATGTCTTCCA-3⬘ and 5⬘-TCTGCCTTGATTCTTACAGGTGAC-3⬘ direct the synthesis of a 500-bp fragment from rat RNA. GAPDH primers 5⬘-ATCACCATCTTCCAGGAGCG-3⬘ and 5⬘-TTGTCATACCAGGAAATGAG-3⬘ were selected based on conserved sequences in the human, rat, and mouse and direct the synthesis of a 720-bp fragment. PCR was performed for 30 cycles, with denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min, with an extra 5-min extension for the last cycle. Aliquots of PCR products (10 l) were separated by electrophoresis on 1.5% agarose gels in 40 mM Tris acetate-1 mM EDTA (pH 8.0). Gels were visualized after ethidium bromide staining under UV transillumination. Gel mobility shift assay. Oligonucleotides to the sense and antisense strands containing putative TEF-1 binding sites were synthesized by an automated solid-phase synthesizer (Gene Assembler Plus, Amersham Pharmacia Biotech) by -cyanoethyl phosphoramidite chemistry. The radiolabeled probe for the gel mobility shift assays was the (⫺84/⫺61) mouse skACT promoter fragment containing the canonical MCAT sequence (17). Cold competitor probes from the S100B promoter were designed encoding MCAT1, a mutant MCAT1mut, MCAT2, and MCAT2mut. These oligonucleotide sequences are shown in Fig. 7B. MCAT1 and MCAT2 sites are mapped on the S100B promoter in Fig. 7A. Nuclear extracts from cardiac myocytes were prepared as described by Kariya et al. (14). Binding reactions were performed as described previously (14, 16, 17). Statistical analysis. Treated-to-control ratios were tested for deviation from unity by calculation of confidence limits. Mean values were compared by analysis of variance by the Fig. 1. Absence of basal S100B mRNA expression in myocardium in vivo and myocardial cells in culture. Adult rats were killed at 35 days after coronary artery ligation (cor. art. lig.). Steady-state levels of S100B and GAPDH mRNAs were determined by RNase protection. The protected fragments specific for rat S100B (264 bp) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 413 bp) mRNAs are shown. The results of a representative experiment include RNA from cultured neonatal (cult. neo.) myocytes and fibroblasts, fetal, neonatal, and adult hearts and brains, and hearts from experimental animals as indicated. Rat tRNA was used as a negative control. AJP-Heart Circ Physiol • VOL 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 rosporine (20 nM), the Ca2⫹ channel blocker nifedipine (1 M), the Ca2⫹ ionophore A-23187 (10 nM), or vehicle diluent (100 M ascorbic acid for NE, PE, Iso, T3, ANG II, CEC, and WB-4101 or 0.01% DMSO for staurosporine, nifedipine, A-23187, and PMA) for 48 h. The cell lysates were assayed for luciferase and CAT activity by following published techniques (7, 11). Cotransfection with any of the plasmids or treatment of cultures as described above did not affect CAT activity. The differences of the respective luciferase and CAT activities between duplicate dishes were ⬍10% of their mean. Luciferase activity was normalized for transfection efficiency on the basis of CAT activity in the same dish. Treated-tocontrol ratios were tested from deviation from unity by calculation of confidence limits. RNase protection assay. RNA was isolated from 1) cultured rat neonatal myocytes and fibroblasts, 2) rat tissues, including the fetal, neonatal, and adult heart and brain, and 3) residual noninfarcted rat left ventricular myocardium outside the territory supplied by the ligated coronary artery (32), with the use of a one-step guanidine thiocyanate-phenol method (6). RNase protection assays to determine steadystate levels of S100B and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNase were performed as previously described (32). Detection of human S100B mRNA by RT-PCR. RNA was isolated from myocyte cultures and human and rat brains by a one-step guanidine thiocyanate/phenol method (6) and pretreated with RNase-free DNase 1 (Boehringer-Mannheim; Laval, Quebec, Canada) before first-strand DNA synthesis with random hexamer primers. Human brain RNA was derived from archival specimens without patient identifiers. The human S100B primers 5⬘-TGGACAATGATGGAGACGG-3⬘ and 5⬘-ATTAGCACAACACGGCTGG-3⬘ direct the syn- H195 H196 ␣1-ADRENERGIC INDUCTION OF S100B IN HEART Student-Newman-Keuls test, with significance defined as P ⬍ 0.05. RESULTS Fig. 2. Induction of the human and rat S100B gene by ␣1-adrenergic stimulation in neonatal rat myocyte cultures transfected with the human S100B gene. Rat (A) and human (B) S100B mRNAs were assayed by RT-PCR in human and rat brain, respectively, and in rat neonatal myocyte cultures transfected with the human S100B gene either untreated (vehicle only) or stimulated with norepinephrine (NE), isoproterenol (Iso), phenylephrine (PE), or 3,5,3⬘-triiodothyronine (T3), as described in MATERIALS AND METHODS. The results of a representative experiment are presented. GAPDH mRNA was used as an internal control for the RT-PCR reaction. The sizes of the RT-PCR products for rat S100B (500 bp), human S100B (210 bp), and GAPDH (720 bp) are indicated. AJP-Heart Circ Physiol • VOL 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 S100B exhibits no basal expression in cultured neonatal rat cardiac myocytes or fibroblasts but is present in residual rat myocardium adjacent to myocardial infarction. Steady-state levels of S100B and GAPDH mRNAs were determined with the use of an RNase protection assay in cultured rat neonatal cardiac myocytes and fibroblasts and in the fetal, neonatal, and adult rat heart and brain as well as in noninfarcted adult myocardium adjacent to an infarct resulting from coronary artery ligation (Fig. 1). S100B mRNA was not detected in cultured neonatal myocytes or fibroblasts or in the fetal, neonatal, and adult heart and not in the fetal or neonatal brain, but it was highly expressed in the adult brain. After coronary artery ligation, S100B mRNA was expressed in the peri-infarct region of the adult myocardium (35 days postinfarction) at levels equivalent to those seen in the adult brain. The constant amounts of GAPDH mRNA in all samples served as controls for the quality of the RNA and confirmed equal loading in all lanes. Coronary artery ligation is associated with increased levels of NE. Thirty-five days after coronary artery ligation, increased NE levels were seen in the periinfarct region of the adult myocardium (35 days postin- farction) compared with sham-operated animals (696 ⫾ 34 vs. 468 ⫾ 25 ng/g left ventricular tissue, respectively, P ⬍ 0.05, n ⫽ 6). Expression of exogenous human and endogenous rat S100B gene in transfected rat cardiac myocytes. Cultured neonatal rat cardiac myocytes were transfected with plasmid pW9Fc carrying 17.3 kb of human genomic DNA that includes a full-length S100B gene. Human and rat S100B mRNAs were assayed by RTPCR. The endogenous rat and human S100B mRNAs were expressed in rat and human brains, respectively (Fig. 2, A and B). The endogenous rat S100B mRNA was absent from untreated rat myocyte cultures and present after treatment with the ␣1-adrenergic agonists NE and PE but not with the 1-agonist Iso or T3 (Fig. 2A). Similarly, the level of the human S100B mRNA derived from the transfected human S100B gene was markedly increased in cultures treated with ␣1-adrenergic agonists NE and PE from a low basal level in untreated cultures (Fig. 2B). Treatment with the 1-adrenergic agonist Iso or T3 did not increase the level of human S100B mRNA above the basal level. ␣1-Adrenergic stimulation specifically activates human S100B promoter through PKC signaling pathways. To study the activation of the human S100B promoter by hormonal stimulation, we initially cloned progressively shorter segments of the maximal human S100B promoter sequentially deleted from the 5⬘ end in ␣1-ADRENERGIC INDUCTION OF S100B IN HEART construct pGBS ⌬⫺162/⫹698 abolished transcription (Fig. 3). In response to hormonal stimulation, transcription from the basic (cloned in pGBS ⫺162/⫹698) and maximal (cloned in pGBS ⫺6,689/⫹698) promoters was stimulated by the ␣1-agonists NE and PE. Treatment with PE stimulated transcription fourfold, equally from the basic and maximal promoters (Fig. 4A). The PE response was blocked with the use of the ␣1Aadrenergic receptor antagonist WB-401 but not the ␣1B-adrenergic receptor antagonist CEC (Fig. 4A). Treatment with Iso, T3, or ANG II did not stimulate transcription from the basic or maximal S100B promoter (Fig. 4B). Transcription from the basic and maximal S100B promoters was also stimulated by PMA, an activator of PKC, and the stimulation of transcription by PE and PMA was blocked by the PKC antagonist staurosporine (Fig. 5A). Neither the calcium ionophore A-23187 nor the calcium channel antagonist nifedipine had any effect on basal or PE-stimulated transcription (Fig. 5A). By using a cotransfection strategy, we found that transcription was stimulated twofold, equally from both promoters by ⌬PKC-, a constitutively active mutant of PKC- (Fig. 5B). This stimulation was blocked by staurosporine. Furthermore, stimulation Fig. 3. Basal activity of human S100B promoter-luciferase (Luc) reporter constructs in neonatal rat cardiac myocytes. The maximal S100B promoter (⫺6,689/⫹698) and progressively shorter fragments sequentially deleted from the 5⬘ end of the maximal promoter were cloned into the promoterless plasmid pGL3 in front of the luciferase reporter gene to generate plasmids pGBS ⫺6,689/⫹698, pGBS ⫺4,463/⫹698, pGBS ⫺782/⫹698 and pGBS ⫺162/⫹698, which are depicted schematically. The plasmid pGBS ⌬⫺162/⫹698 was generated similarly from a fragment created by deleting the region ⫺162/⫹698 from pGBS ⫺4,463/⫹698. The relative luciferase activity of each plasmid after transfection into neonatal rat myocyte cultures is shown on the right of each plasmid. Values are the mean of six independent experiments, with duplicate dishes in each experiment. The base sequence numbering is expressed relative to the transcription initiation site (⫹1), in accordance with the human S100B genomic sequence in the National Center for Biotechnology Information database. Exon 1 is illustrated as an open bar and the luciferase gene as an arrow. The restriction enzyme sites are indicated as follows: H, HindIII; B, BamH I; K, KpnI; P, PvuII; S, SmaI. AJP-Heart Circ Physiol • VOL 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 front of the luciferase reporter gene. These constructs were transfected into cultured neonatal rat cardiac myocytes. We showed that basal transcription from the various S100B promoter constructs was selective for cardiac myocytes because it was absent in similarly transfected cultured neonatal rat fibroblasts (data not shown). We also identified a basic promoter cloned in pGBS ⫺162/⫹698 and defined as the shortest S100B promoter fragment assayed, which spanned 162 bp upstream of the transcription initiation site, 71 bp of exon 1, and 627 bp of intron 1 (Fig. 3). The basic promoter contains several general transcription elements, including a TATA box, a reverse CCAAT box, and two GC boxes (1). The relative luciferase activities of the various cloned constructs that comprised additional sequences upstream of the basic promoter were 2-fold for pGBS ⫺782/⫹698, 0.5-fold for pGBS ⫺4,463/⫹698, and 4-fold for the maximal promoter ⫺6,689/⫹698, all relative to the basic promoter (Fig. 3). This indicates the presence of positive regulatory elements in the sequences ⫺782/⫺162 and ⫺6,689/ ⫺4,463 and of a negative regulatory element in the sequence ⫺4,463/⫺782. There were no additional transcription initiation sites upstream of the basic promoter, as absence of the basic promoter from the cloned H197 H198 ␣1-ADRENERGIC INDUCTION OF S100B IN HEART DISCUSSION Fig. 4. ␣1-Adrenergic stimulation specifically activates the basic and maximal human S100B promoters through the ␣1A-adrenergic receptor. Neonatal rat cardiac myocytes were transfected with pGBS ⫺162/⫹698 (basic S100B promoter) or pGBS ⫺6,689/⫹698 (maximal S100B promoter). A: transfected myocytes were treated with ␣1adrenergic agonists NE or PE, the ␣1A-adrenergic receptor antagonist WB-4101 (WB) or the ␣1B-adrenergic receptor antagonist chloroethyl clonidine (CEC), or with PE in the presence of WB or CEC and assayed for luciferase activity, which is expressed relative to myocytes treated with vehicle as described in MATERIALS AND METHODS. B: transfected myocytes were treated with Iso, T3, or ANG II and assayed for luciferase activity, as described above. Bars are the means ⫾ SE of luciferase expression from 6 independent experiments, with duplicate dishes in each experiment. * P ⬍ 0.05 vs. myocytes treated with vehicle and assigned a value of 1. AJP-Heart Circ Physiol • VOL The postinfarct hypertrophic response in the myocardium is initiated by multiple trophic signals. The state of local and systemic sympathetic hyperactivity that accompanies myocardial infarction contributes one of these signals through ␣1-adrenergic stimulation of the ␣1A-adrenergic receptor (12, 18, 29). Intracellular second messengers for signal transduction triggered by ␣1-adrenergic stimulation include Ca2⫹ and PKC (23). The latter signaling pathway involves PKC- and possibly other isoforms of the enzyme (␣, ␦, ⑀, and ) that have been shown to be present in the rat heart (2, 28). Distal components of the PKC signaling pathway include TEF-1, RTEF-1, Fos, and Jun (15, 17, 19, 31). Phosphorylation of these proteins by PKC is one of the control mechanisms that influences their 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 with ⌬PKC-, when combined with PE treatment, did not further increase the stimulation obtained with PE alone (Fig. 5B). TEF-1 and RTEF-1 modulate transcription from human S100B promoter through elements located upstream of basic promoter. Cotransfection with TEF-1 or RTEF-1, which are distal components of the PKC signal transduction pathway, did not influence transcription from the basic promoter (⫺162/⫹698) or the sequence ⫺782/⫹698, but influenced transcription from the upstream sequences ⫺4,463/⫹698, and ⫺6,689/ ⫹698 implicating the presence of MCAT elements located in the upstream sequences. The effects of TEF-1 and RTEF-1 on transcription from the upstream sequences were different. Whereas TEF-1 inhibited basal transcription and reversed the transcriptional stimulation mediated by PE (Fig. 6A), RTEF-1-stimulated basal transcription 1.5- to 2-fold and had no effect on the PE-induced transcriptional stimulation (Fig. 6B). Cotransfection with the transcription factors Fos or Jun, which are also distal components of the PKC signaling pathway, did not influence transcription from either the basic or maximal promoter (data not shown). S100B MCAT elements exhibit specific binding to cardiac myocyte nuclear extracts. Two MCAT motifs were identified in the maximal S100B promoter, MCAT1 (⫺1,984/⫺1,978) and MCAT2 (⫺1,333/⫺1,327) (Fig. 7A). To determine whether the S100B MCAT sequences bound members of the TEF-1 family, gel mobility shift assay was performed with the use of cardiac myocyte nuclear extract and the radiolabeled ⫺84/⫺61 skACT promoter containing the canonical MCAT sequence (Fig. 7B). The interaction of TEF-1 with the skACT MCAT produced a band pattern (lane 1) that was competed out by excess unlabeled MCAT sequences of the skACT promoter (lane 2) and MCAT1 (lane 3) and MCAT2 (lane 5) of the S100B promoter. Demonstrating specificity, mutated MCAT1 (lane 4) or MCAT2 (lane 4) did not compete out this binding. These results suggest that the MCAT1 and MCAT2 elements within the S100B promoter can interact with members of the TEF-1 family in cardiac myocytes. ␣1-ADRENERGIC INDUCTION OF S100B IN HEART transactivation or transrepression of specific promoters (13, 34). TEF-1 and RTEF-1 belong to a family of eukaryotic transcription factors that share sequence homology in the tetraethylammonium (or ATTS) DNA binding domain (13). In mammalian promoters, TEF-1 and RTEF-1 bind to M-CAT elements (9, 19). It is possible that these two transcription factors play selective opposing roles in the regulation of fetal gene induction during the hypertrophic response induced by ␣1-adrenergic stimulation in cardiac myocytes. This H199 Fig. 5. ␣1-Adrenergic stimulation activates the basic and maximal human S100B promoters through the protein kinase C (PKC) signaling pathway. A: neonatal rat cardiac myocytes were transfected with pGBS ⫺162/⫹698 (basic S100B promoter) or pGBS ⫺6,689/ ⫹698 (maximal S100B promoter). Transfected myocytes were treated with PE, the Ca2⫹ ionophore A-23187 (Ion), the Ca2⫹ channel blocker nifedipine (Nif), the PKC antagonist staurosporine (Stau), or phorbol 12-myristate 13-acetate (PMA), or with NE in the presence of Ion, Nif, or Stau, and PMA ⫹ Stau, and assayed for luciferase activity, which is expressed relative to myocytes treated with vehicle, as described in MATERIALS AND METHODS. B: neonatal rat cardiac myocytes were transfected with pGBS ⫺162/⫹698 (basic S100B promoter) or pGBS ⫺6,689/⫹698 (maximal S100B promoter), or cotransfected with the above promoters and ⌬PKC- (a constitutively active mutant of PKC-). Control myocytes were cotransfected with the plasmid vector SR␣. The transfected or cotransfected plasmids were treated with PE or Stau and assayed for luciferase activity, which is expressed relative to myocytes transfected with the plasmid vector SR␣ and treated with vehicle as described in MATERIALS AND METHODS. Bars represent the means ⫾ SE of luciferase expression from six independent experiments, with duplicate dishes in each experiment. * P ⬍ 0.05 vs. control myocytes assigned a value of 1. AJP-Heart Circ Physiol • VOL 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 possibility is based on the observations that RTEF-1 transactivates the promoters of the fetal genes -MHC and skACT, whereas TEF-1 downregulates transcription from the skACT promoter in these cells (31). The results of our previous studies implicated S100B as a negative modulator of the hypertrophic response contributed by ␣1-adrenergic stimulation (32, 33). We were able to demonstrate that S100B inhibited the induction of -MHC and skACT through the PKC pathway in the context of the hypertrophic response induced by ␣1-adrenergic stimulation in cultured rat myocytes (32). The present study was undertaken to examine whether the S100B gene induction in cardiac muscle was mediated by the ␣1-adrenergic pathway. This would support the hypothesis that S100B functions as a feedback regulator of this component of the hypertrophic response. We (33) reported a de novo presence of S100B protein and mRNA in the peri-infarct region of human and rat hearts. In the present study, we found that the presence of S100B mRNA in the peri-infarct region of the rat heart after an experimentally provoked myocardial infarction consequent to coronary artery ligation coincided with increased left ventricular NE levels. We planned to demonstrate that the induction of steadystate S100B mRNA levels from undetectable in normal heart to a level equivalent to that seen in the rat brain after myocardial infarction was due to an induction of the S100B gene by increased NE levels. For this purpose, we first transfected cultured neonatal rat cardiac myocytes with a full-length human S100B gene and showed that both the endogenous rat S100B gene and the transfected human S100B gene were specifically induced by the ␣1-adrenergic agonists NE and PE but not the 1-adrenergic agonist Iso or T3. To begin to dissect the functional activation of the human S100B promoter in the heart, we transfected cultured rat myocytes with constructs incorporating a luciferase reporter gene driven by the human S100B promoter. In the first instance, we used a maximal H200 ␣1-ADRENERGIC INDUCTION OF S100B IN HEART S100B promoter, spanning 6,689 bp upstream of the transcription initiation site, and progressively shorter fragments sequentially deleted from the 5⬘ end of this promoter, to define a basic promoter essential for transcription (spanning 162 bp upstream of the transcrip- Fig. 6. Transcription enhancer factor-1 (TEF-1) or related to TEF-1 (RTEF-1) modulate transcription from the human S100B promoter through elements located upstream of the basic promoter. Neonatal rat cardiac myocytes were transfected with pGBS ⫺162/⫹698, pGBS ⫺782/⫹698, pGBS ⫺4,463/⫹698, or pGBS ⫺6,689/⫹698 or cotransfected with the above promoters and TEF-1 (A) or RTEF-1 (B). Control myocytes were cotransfected with the plasmid vector pXJ40. The transfected or cotransfected myocytes were treated with PE and assayed for luciferase activity, which is expressed relative to myocytes transfected with the plasmid vector pXJ40 and treated with vehicle as described in MATERIALS AND METHODS. Bars represent means ⫾ SE of luciferase expression from six independent experiments, with duplicate dishes in each experiment. * P ⬍ 0.05 vs. control myocytes assigned a value of 1. AJP-Heart Circ Physiol • VOL 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 tion initiation site), two positive regulatory elements (located in the regions ⫺782/⫺162 and ⫺6,689/ ⫺4,463), and one negative regulatory element (located in the region ⫺4,463/⫺782). There was no transcription from any of the S100B promoter fragments in cultured neonatal rat cardiac fibroblasts. In our previous studies, in cultured neonatal rat astrocytes, we used a similar strategy starting with a maximal promoter that spanned 4,463 bp upstream of the transcription initiation site (3). In these studies, we identified the same basic promoter, the positive regulatory element at ⫺782/⫺162, and the negative regulatory element at ⫺4,463/⫺782. Presently, by using a longer maximal promoter as our starting point for generating the 5⬘ deletion fragments, we extended these results by identifying an additional positive regulatory element at ⫺6,689/⫺4,463. The similarity in the basic human S100B promoter and upstream regulatory elements identified in rat astrocytes and myocytes, and the absence of transcription in rat fibroblasts, indicate a tissue-selective distribution of specific transcription factors that activate the S100B promoter and are conserved among mammalian species. This is consistent with the experimental evidence suggesting highly specialized functional roles for S100B in the mammalian brain and heart (8). We then used the basic and maximal S100B promoters to study the induction of S100B in cardiac myocytes by ␣1-adrenergic stimulation. Both promoters were activated by the ␣1-adrenergic agonists NE and PE through the ␣1A-adrenergic receptor, but not by any other hormonal myocardial trophic signals, including T3, ANG II, or the 1-adrenergic agonist Iso. The activation by PE was blocked by the PKC antagonist staurosporine suggesting involvement of the PKC signaling pathway. This suggestion was supported by showing activation of the S100B promoter by treatment with PMA (which activates PKC) and by cotransfection with ⌬PKC-, a constitutively active mutant of PKC-. The activation by PMA and ⌬PKC- was also blocked by staurosporine again implicating the PKC signaling pathway. The twofold activation seen with ⌬PKC was approximately one-half of that seen with PE and the two together were not additive, suggesting that both PKC- and other isoforms of PKC are involved in this activation consistent with previously published results (2, 28). There were no significant differences in the ␣1-ADRENERGIC INDUCTION OF S100B IN HEART H201 activation of the S100B promoter by PE in the presence or absence of either the Ca2⫹ ionophore A-23187 or the Ca2⫹ channel blocker nifedipine, suggesting that Ca2⫹ was not a likely second messenger of the ␣1-adrenergic signal leading to the activation of the S100B promoter. Taken together, the above data indicate that the same ␣1-adrenergic pathway that initiates and sustains the hypertrophic response in cardiac myocytes by activating PKC and is subject to negative modulation by S100B also induces the S100B gene (32). This supports the hypothesis that S100B is a negative feedback regulator of this pathway. Whereas ␣1-adrenergic activation of the S100B promoter proceeded equally well with the basic and maximal promoters, the same was not the case for the transcription factors TEF-1 and RTEF-1. Both factors required the maximal promoter for their action, consistent with the presence of MCAT sequences (CATTCCT at ⫺1,333 to ⫺1,327 and CATTCCA at ⫺1,984 to ⫺1,978) upstream of the basic promoter that exhibited specific binding activity. TEF-1 transrepressed the maximal full-length S100B promoter under basal conditions and with PE, thereby preventing ␣1-adrenergic AJP-Heart Circ Physiol • VOL activation of the S100B promoter that was previously documented. The inhibition of the S100B promoter was not seen with 10-fold and 100-fold lower concentrations of TEF-1 (data not shown). The inhibitory effect of TEF-1 is comparable to the response of the skACT promoter and may result from TEF-1 overabundance sequestering or “squelching” a limiting cofactor (31). Conversely, RTEF-1 transactivated the same S100B promoter twofold versus the fourfold activation seen with PE, and the two together were not additive. This suggests that a component of the ␣1-adrenergic induction of the S100B promoter is mediated in part through RTEF-1. It is conceivable that this two-tiered mechanism of induction of S100B confers the following advantages. First, the RTEF-1 component provides a means to regulate the induction of the S100B gene coordinately with the induction of fetal genes such as -MHC and skACT, and, second, the non-RTEF-1 component ensures an induction of the S100B gene in conditions where the amount of RTEF-1 might be limiting. Moreover, because PE alone activated the basic and maximal S100B promoters equally well, the above results also suggest that in the presence of the maxi- 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 Fig. 7. The S100B MCAT binds myocyte TEF-1. A: schematic representation of the ⫺6,698/⫹698 S100B promoter fragment containing the first exon and a portion of the first intron. The location of the restriction sites used to generate the series of 5⬘ promoter deletions and of the MCAT, GATA4, MEF-2, and TATA sequences is indicated. See Fig. 3 for restriction enzyme site designations. B: endogenous TEF-1 in cardiac myocyte nuclear extracts binds to the S100B MCAT elements. A double-stranded oligonucleotide of the mouse ⫺84/⫺61 skACT promoter sequence, containing the canonical MCAT sequence, was end labeled with 32P and incubated with 10 g of cardiac myocyte nuclear extract. Free and complex probes were separated on a 6% native gel (bottom). Arrows indicate the position of free and the shifted TEF-1-DNA complex. Gel mobility shift assay was done with no competitor (lane 1), with 100-fold molar excess of unlabeled ⫺84/⫺61 skACT promoter fragment (lane 2), and with 100-fold molar excess of unlabeled S100B, MCAT1, and MCAT2 fragments (lanes 3 and 5) and their mutated versions (lanes 4 and 6). Sequences of the oligonucleotides are shown at the top. Binding sites are underlined and boldface. Lowercase letters identify mutated bases. H202 ␣1-ADRENERGIC INDUCTION OF S100B IN HEART We thank C. McMahon, A. Haddad, and J. Culbert for excellent technical assistance and Drs. L. R. Karns, P. C. Simpson, A. F. R. Stewart, and I. Verma for the gift of the essential expression plasmids and for helpful discussions. This work was supported in part by the Canadian Institutes of Health Research. REFERENCES 1. Allore RJ, Friend WC, O’Hanlon D, Neilson KM, Baumal R, Dunn RJ, and Marks A. Cloning and expression of the human S100B gene. J Biol Chem 265: 15537–15543, 1990. 2. Bogoyevitch MA, Parker PJ, and Sugden PH. Characterization of protein kinase C isotype expression in adult rat heart. Protein kinase C-epsilon is a major isotype present, and is activated by phorbol esters, epinephrine, and endothelin. Circ Res 72: 757–767, 1993. 3. Castets F, Griffin WST, Marks A, and Van Eldik LJ. Transcriptional regulation of the human S100B gene. Mol Brain Res 46: 208–216, 1997. 4. Chien KR, Knowlton KU, Zhu H, and Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J 5: 3037–3046, 1991. 5. Chien KR, Zhu H, Knowlton KU, Miller-Hance W, Van Bilsen M, O’Brien TX, and Evans S. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol 55: 77–95, 1993. 6. Chomczynski P and Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. 7. De Wet JR, Wood KV, DeLuca M, Helinski DR, and Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol 7: 725–737, 1987. 8. Donato R. S100: a multigene family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 33: 637–668, 2001. AJP-Heart Circ Physiol • VOL 9. Farrance IKG, Mar JH, and Ordahl CP. M-CAT binding factor is related to the SV40 enhancer binding factor, TEF-1. J Biol Chem 267: 17234–17240, 1992. 10. Friend WC, Clapoff S, Landry C, Becker LE, O’Hanlon D, Allore RJ, Brown IR, Marks A, Roder J, and Dunn RJ. Cell-specific expression of high levels of human S100 in transgenic mouse brain is dependent on gene dosage. J Neurosci 12: 4337–4346, 1992. 11. Gorman CM, Moffat LF, and Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol 2: 1044–1051, 1982. 12. Itagaki T, Toma Y, Umemoto S, Yamakawa K, Yoshinaga T, Fukuta S, and Kusukawa R. Hemodynamic benefits of long-term bunazosin (␣-1 blocker) therapy in rats with myocardial infarction and heart failure. Jpn Circ J 53: 313–318, 1989. 13. Jiang SW, Dong M, Trujillo MA, Miller LJ, and Eberhardt NE. DNA binding of TEA/ATTS domain factors is regulated by protein kinase C phosphorylation in human choriocarcinoma cells. J Biol Chem 276: 23464–23470, 2001. 14. Kariya K, Ferrance IKG, and Simpson PC. Transcriptional enhancer factor-1 in cardiac myocytes interacts with an ␣1adrenergic- and protein kinase C inducible element in the rat -myosin heavy chain promoter. J Biol Chem 268: 26658–26662, 1993. 15. Kariya K, Karns LR, and Simpson PC. Expression of a constitutively activated mutant of the -isozyme of protein kinase C in cardiac myocytes stimulates the promoter of the -myosin heavy chain isogene. J Biol Chem 266: 10023–10026, 1991. 16. Kariya K, Karns LR, and Simpson PC. An enhancer core element mediates stimulation of the rat -myosin heavy chain promoter by an ␣1-adrenergic agonist and activated -protein kinase C in hypertrophy of cardiac myocytes. J Biol Chem 269: 10023–10026, 1994. 17. Karns LR, Kariya K, and Simpson PC. M-CAT, CArG, and Sp1 elements are required for ␣1-adrenergic induction of the skeletal ␣-actin promoter during cardiac myocyte hypertrophy. Transcriptional enhancer factor-1 and protein kinase C as conserved transducers of the fetal program in cardiac growth. J Biol Chem 270: 410–417, 1995. 18. Knowlton KU, Michel MC, Itani M, Shubeita HE, Ishihara K, Brown JH, and Chien KR. The ␣1A-adrenergic receptor subtype mediates biochemical, molecular, and morphologic features of cultured myocardial cell hypertrophy. J Biol Chem 268: 15374–15380, 1993. 19. Larkin SB and Ordahl CP. Multiple layers of control in transcriptional regulation by M-CAT elements and the TEF-1 protein family. In: Heart Development, edited by Rosenthal N and Harvey RP. San Diego, CA: Academic, 1998, p. 307–329. 20. Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, and Molkentin JD. The transcriptional factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem 276: 30245–30253, 2001. 21. Long CS, Henrich CJ, and Simpson PC. A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regul 2: 1081–1085, 1991. 22. Morimoto T, Hasegawa K, Kaburagi S, Kakita T, Wada H, Yanazume T, and Sasayama S. Phosphorylation of GATA-4 is involved in ␣1-adrenergic agonist-responsive transcription of the endothelin-1 gene in cardiac myocytes. J Biol Chem 275: 13721– 13726, 2000. 23. Nishizuka Y. Studies and perspectives of protein kinase C. Science 233: 305–312, 1986. 24. Orenstein TL, Parker TG, Butany JW, Goodman JM, Dawood F, Wen WH, Wee L, Martino T, McLaughlin PR, and Liu PP. Favorable left ventricular remodeling following large myocardial infarction by exercise training. Effect on left ventricular morphology and gene expression. J Clin Invest 96: 858–866, 1995. 25. Parker TG. Molecular biology of cardiac growth and hypertrophy. Herz 18: 245–255, 1993. 26. Parker TG and Schneider MD. Growth factors, proto-oncogenes, and plasticity of the cardiac phenotype. Annu Rev Physiol 53: 179–200, 1991. 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 mal promoter (as is the case in the physiological situation), ␣1-adrenergic stimulation of S100B is differentially modulated by TEF-1 and RTEF-1. Additional regulation may be dependent on elements present in the basic promoter, first intron, and/or upstream of the basic promoter. Candidate elements include multiple MEF-2 and GATA4 sites (Fig. 7A) that were reported (20, 22, 36) to be targets for ␣1-adrenergic induction of ␣-MHC, B-atrial natriuretic factor, myosin light chain, and cardiac troponin C promoters. The opposing actions of TEF-1 and RTEF-1 on transcription from the S100B promoter are consistent with their possible opposing roles in the expression of the genetic program that underlies postinfarct myocardial hypertrophy (31). Furthermore, the transrepression of the S100B promoter by TEF-1 may also be involved in silencing this gene in normal myocardium. Thus the relative abundance of TEF-1 and RTEF-1 or their selective inactivation or activation, respectively, may control the induction of the S100B gene during myocardial hypertrophy. In the postinfarct setting, the initial accession and subsequent waning of the hypertrophic response are necessary for orderly ventricular remodeling (27). This likely requires a fine balance between signaling pathways, such as ␣1-adrenergic stimulation to initiate and sustain the hypertrophic response, and their negative feedback regulators, such as S100B. Our results provide evidence for mechanisms of control of induction of the S100B gene that may be important for maintaining this balance. ␣1-ADRENERGIC INDUCTION OF S100B IN HEART AJP-Heart Circ Physiol • VOL 32. Tsoporis JN, Marks A, Kahn HJ, Butany JW, Liu PP, O’Hanlon D, and Parker TG. S100 inhibits ␣1-adrenergic induction of the hypertrophic phenotype in cardiac myocytes. J Biol Chem 272: 31915–31921, 1997. 33. Tsoporis JN, Marks A, Kahn HJ, Butany JW, Liu PP, O’Hanlon D, and Parker TG. Inhibition of norepinephrineinduced cardiac hypertrophy in S100 transgenic mice. J Clin Invest 102: 1609–1616, 1998. 34. Ueyama T, Zhu C, Valenzuela YM, Suzow JG, and Stewart AFR. Identification of the functional domain in the transcription factor RTEF-1 that mediates ␣1-adrenergic signaling in hypertrophied cardiac myocytes. J Biol Chem 275: 17476–17480, 2000. 35. Waspe LE, Ordahl CP, and Simpson PC. The cardiac -myosin heavy chain isogene is induced selectively in ␣1-adrenergic receptor-stimulated hypertrophy of cultured rat heart myocytes. J Clin Invest 85: 1206–1214, 1990. 36. Zhu H, Nguyen VTB, Brown AB, Pourhosseni A, Garcia AV, Bilsen MV, and Chien KR. A novel, tissue-restricted zinc finger protein (HF-1b) binds to the cardiac regulatory element (HF-1b/MEF-2) in the rat myosin light-chain 2 gene. Mol Cell Biol 13: 4432–4444, 1993. 284 • JANUARY 2003 • www.ajpheart.org Downloaded from http://ajpheart.physiology.org/ by 10.220.33.4 on June 12, 2017 27. Pfeffer MA, Braunwald E, Moye LA, Basts L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamar GA, Packer M, Rouleau JL, Rutherford J, Wertheimer JH, and Hawkins CM. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 327: 669–677, 1992. 28. Roman BB, Geenen DL, Leitges M, and Buttrick PM. PKC- is not necessary for cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280: H2264–H2270, 2001. 29. Sigurdsson A, Held P, and Swedberg K. Short- and longterm neurohormonal activation following acute myocardial infarction. Am Heart J 126: 1068–1076, 1993. 30. Simpson PC. Stimulation of hypertrophy of cultured neonatal rat heart cells through an ␣1-adrenergic receptor and induction of beating through an ␣1- and 1-adrenergic receptor interaction. Circ Res 56: 884–894, 1985. 31. Stewart AFR, Suzow J, Kubota T, Ueyama T, and Chen HH. Transcription factor RTEF-1 mediates ␣1-adrenergic reactivation of the fetal gene program in cardiac myocytes. Circ Res 83: 43–49, 1998. H203