* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biology 1406 Exam 2
Survey
Document related concepts
Metalloprotein wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Citric acid cycle wikipedia , lookup
Electron transport chain wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Transcript
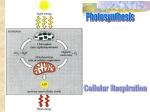
Biology 1406 Exam 2 - Metabolism Chs. 5, 6 and 7 energy - capacity to do work 5.10 kinetic energy - energy of motion : light, electrical, thermal, mechanical potential energy - energy of position or “stored energy” : chemical, mechanical * Is chemical energy potential or kinetic energy? The position of what is storing energy? Thermodynamics - the study of energy transformations. Two of the laws important to understanding living organisms first law - conservation of energy; amount of energy in the universe is constant, with energy not created or destroyed (can change form) second law - energy transformations increase entropy in the universe - all energy will be “scattered heat” * What is entropy? * Can any part of the universe decrease entropy? * Does life violate the second law of thermodynamics? Chemical reactions can be classified: 5.11 exergonic - release energy; products contain less energy than reactants endergonic - require an input of energy; products contain more energy than reactants * Is energy created in endergonic reactions or destroyed in exergonic reactions? anabolic - larger molecules are produced from smaller molecules catabolic - larger molecules are made into smaller molecules Adenosine triphosphate (ATP) is key molecule in linking exergonic reactions to endergonic reactions in cells. This allows energy to be captured and applied to work in the cells. 5.12, 6.4 ATP can be converted to ADP (adenosine diphosphate) and to AMP (adenosine monophosphate) in a completely reversible reaction : Energy Energy ATP <-------------> ADP + P <--------------> AMP + P + P Energy Energy *Know basic structure of ATP and within the structure which chemical bonds store energy for use in cell work. *What is the role of ATP in cellular metabolism? Enzymes are proteins that catalyze chemical reactions 5.13-5.16 - control rate of chemical reaction by lowering activation energy - do not alter reaction or provide energy - are not used up in reaction - are very specific, catalyzing only one or a few reactions enzymes work because of their three-dimensional shape that includes active site - active site complementary to substrate molecule - orients reactant molecule(s) and stresses bonds - cofactors give enzyme correct shape - inhibitors give enzyme incorrect shape, block action - competitive - noncompetitive - can bind reversibly or nonreversibly - environmental factors can inhibit enzymes by changing shape - pH, heat, salts Enzymes frequently work as a series in a metabolic pathway - reactions occur in an orderly sequence - enzymes of a metabolic pathway may be attached to membranes or isolated in membrane compartment - metabolic pathways are often regulated by the final product of the last enzyme being a reversible inhibitor for the first enzyme * What is the benefit of having the enzymes of a metabolic pathway attached to a membrane in sequence? Electron carriers (also called hydrogen carriers) hold and transport high-energy electrons 6.5 - NAD+ (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide) - link oxidation and reduction in redox reactions - temporarily accept high-energy electrons and hydrogen (reduced) - donate high-energy electrons and hydrogen to another molecule (oxidized) - oxidation-reduction reactions involve the transfer of electrons or hydrogen from one molecule or atom to another - oxidation and reduction always occur together - must have an electron donor and an electron acceptor - molecule that donates an electron is oxidized, molecule that accepts electron is reduced Cellular respiration is main energy producing pathway in living organisms - uses glucose as fuel (other molecules can be used) in overall catabolic, exergonic, oxidation/reduction series of reactions - involves three different metabolic pathways 6.1-6.3 1. glycolysis 6.6 - 6.7 - glucose (6 carbon molecule) catabolized to pyruvate (3 carbon molecule) - enzymes of metabolic pathway in cytoplasm of cell, not enclosed in membrane - does not require oxygen - same enzymes (and genes) in all living organisms - “metabolic relict” - produces two ATP molecules per glucose molecule (substrate phosphorylation) - glucose, (2) ADP + P and (2) NAD+ enter and (2) pyruvate, (2) ATP and (2) NADH exit anaerobic respiration (oxygen not used) is glycolysis and fermentation - high-energy electrons to some acceptor other than oxygen - sulfur or nitrogen in some primitive bacteria - pyruvate in many organisms (a process called fermentation) - inefficient, producing only 2 ATP molecules per glucose molecule fermentation is method of removing high-energy electrons from the cell 6.13 -alcohol fermentation in some organisms reduces pyruvate to CO2 and ethanol and oxidizes NADH to NAD+ - lactic acid fermentation in many animals reduces pyruvate to lactic acid and oxidizes NADH to NAD+ Structure of mitochondria: 4.13 matrix is inside inner membrane inner membrane outer membrane intermembrane space is between membranes 2. citric acid cycle (also called TCA cycle or Krebs cycle) 6.6, 6.8 - 6.9 - pyruvate catabolized to carbon dioxide (1 carbon molecule) - enzymes of metabolic pathway in matrix of mitochondria - indirectly requires oxygen (occurs only with oxidative phosphorylation) - produces two ATP molecules per glucose molecule (substrate phosphorylation) - (2) pyruvate, (2) ADP + P and (10) NAD+ enter and (6) CO2, (2) ATP and (10) NADH exit 3. oxidative phosphorylation 6.6, 6.10 - 6.11 - includes electron transport chain and chemiosmosis - uses high-energy electrons from electron carriers - enzymes of ETC and ATP synthase in inner membrane of mitochondria - requires oxygen as final electron acceptor - produces about 34 ATP molecules per glucose - ETC is series of enzymes that accept high-energy electrons from NADH - electrons passed along chain to oxygen, losing small amount of energy each step - energy used to pump hydrogen (H+) from matrix into intermembrane space - H+ becomes more concentrated (100 times) in intermembrane space as in matrix - H+ concentration gradient drives chemiosmosis - chemiosmosis is process of producing ATP from ADP + P by an enzyme complex called the ATP synthase - powered by the flow of H+ from the intermembrane space into the matrix - oxygen, low-energy electrons and H+ combine to form water - (6)oxygen, (12) NADH and (34) ADP + P enter and (6) water, (12) NAD+ and (34) ATP exit aerobic respiration (oxygen used) - glycolysis, citric acid cycle and oxidative phosphorylation - oxygen is final electron acceptor, last two steps occur only in mitochondria - much more efficient, produces about 36 ATP molecules per glucose molecule Summary equation for aerobic cellular respiration: C6H12O6 + 6 O2 ----------> 6 CO2 + 6 H2 O + energy glucose + oxygen -------> carbon dioxide + water + energy in ATP bonds & heat * As the Earth began to accumulate an oxygen atmosphere about three to two billion years ago, what is a possible advantage of a primitive anaerobic cell capturing bacteria that had the metabolic pathway for oxidative phosphorylation (the ancestor of mitochondria)? Carbohydrates, fats and proteins can be catabolized in the cellular respiration metabolic pathways by being converted to intermediaries 6.15 The intermediaries of the cellular respiration pathways can be used as starting points for biosynthesis to produce new molecules for growth 6.16 Photosynthesis Ch 7 - series of metabolic pathways that produce organic molecules - “light formed” - light energy captured in chemical bonds of glucose - overall an endergonic, anabolic, oxidation- reduction series of reactions - occurs only in a group of ancient bacteria and their descendents that formed a symbiotic partnership with a eukaryotic cell (the chloroplast in algae and plants) 4.16 (16.17) - most living organisms on Earth feed on organic molecules from photosynthesis Structure of chloroplast: 7.2, 4.14 - outer and inner membrane - stroma is fluid filling - thylakoid membrane forms interconnected flattened membrane sacs called thylakoids - concentrated hydrogen stored inside thylakoids - light-trapping photosystems, electron transport chain and ATP synthase built into thylakoid membrane - “light reactions” - Calvin cycle enzymes in stroma produce glucose from carbon dioxide - “dark reactions” Nature of light energy 7.6 - radiation or electromagnetic energy - has properties of wave, measured as wavelength - has properties of package of energy, a photon - shorter wavelengths have higher energy photons - visible light (380 - 750 nm wavelength) used in photosynthesis, mostly red and blue wavelengths Photosystems are clusters of photopigments that absorb and trap light energy 7.7 - each photopigment absorbs photon energy of a particular wavelength - chlorophyll a, b, c, d and carotenoids in a photosystem - photon energy absorbed is passed to central chlorophyll a and electron acceptor in the reaction center - light energy converted to chemical energy in excited high-energy electrons - two types of photosystems embedded in thylakoid membrane, both required - photosystem I passes high-energy electrons to an electron carrier, NADP+ - replaces its electrons from electron transport chain - photosystem II passes high-energy electrons to the electron transport chain - replaces its electrons from a water molecule Photosynthesis occurs in two stages: 7.5, 7.8 - 7.11 Light reactions - occur in thylakoid membrane, directly powered by light energy - produce two high energy molecules, ATP and NADPH - photosystem II absorbs light energy and produces high-energy electrons - water is broken apart for electrons ( HOH ---> 2 e-- + H+ + O ) - high-energy electrons pass down electron transport chain to photosystem I - energy from high-energy electrons used by ETC enzymes to pump H+ into interior of thylakoids, produces concentrated H+ - photosystem I absorbs light energy and produces high-energy electrons - these electrons are passed to electron carrier ( NADP+ -----> NADPH ) - chemiosmosis produces ATP ( ADP + P -----> ATP ) - H+ flows through ATP synthase in thylakoid membrane - called photophosphorylation light energy + HOH + NADP+ + ADP+P ------> O2 + NADPH + ATP Calvin cycle (dark reactions, carbon fixation) - enzymes of Calvin cycle metabolic pathway in stroma of chloroplasts - powered by ATP and NADPH from light reactions - produces glucose (organic molecules) from carbon dioxide (CO2) CO2 + NADPH + ATP -------> glucose + NADP+ + ADP + P Summary equation for photosynthesis: light energy + HOH + CO2 ---------> glucose + O2 Regulation of photosynthesis 7.11 - several environmental factors affect rate of photosynthesis: - light intensity and temperature - availability of carbon dioxide and water may be limiting - photorespiration occurs when oxygen builds up in leaves - due to water shortage which closes stomata - oxygen combines with Calvin cycle enzymes preventing glucose production - two groups of plants have evolved adaptations to prevent photorespiration - C4 plants (tropical grasses) and CAM plants (succulents like cacti) *What conditions cause photorespiration? *Describe the adaptations that allow some plants to avoid photorespiration. Evolution of metabolic diversity: - ATP first energy source for primitive cells, produced abiotic - glycolysis first metabolic pathway - used abiotic produced organic molecules to rebuild ATP - chemiosmosis to produce ATP from electron energy - proton pump to eliminate H+ from cell, requires ATP - electron transport chain to remove high-energy electrons from cell - combines ETC to concentrate H+ and proton pump to produce ATP - photosynthesis to build organic molecules using light energy - H2S as electron source first then H2O - sulfur or oxygen released as byproduct, produced oxygen atmosphere - aerobic respiration, using oxygen as final electron acceptor - much more efficient than anaerobic respiration - eukaryotic cells, which combined several primitive prokaryotes into symbiotic cell (mitochondria and chloroplast as cell organelles) Biology 1407 Exam 3 Review Chapters 5,6 and 7 in text Distinguish between kinetic and potential energy. Chemical energy is which of these? Where, exactly, is the energy of chemical energy stored within the molecular structure? Distinguish between exergonic and endergonic and between catabolic and anabolic reactions. What is the role of adenosine triphosphate (ATP) in metabolism? Where within the ATP molecule is energy stored? How do AMP + P + P, ADP + P and ATP compare? What are enzymes and what is their role in metabolism? How do cofactors and inhibitors affect enzymes? What are metabolic pathways? How are they important compared to single enzymes? What object is moved from one atom or molecule to another atom or molecule in an oxidation-reduction reaction? Which molecule is oxidized in this reaction, the donor or the acceptor? What is the role of hydrogen (sometimes called electron) carriers such as NAD+ in metabolism? Write the summary equation for cellular respiration. Overall, is cellular respiration exergonic or endergonic, catabolic or anabolic, oxidation or reduction? What are the three steps, in order, of cellular respiration and where does each occur in the cell? Which of these require the presence of oxygen? Which occur in aerobic cellular respiration? How many ATP are made in each of the three steps? List 3 reactants that go into glycolysis and 3 products that come out. Which have been oxidized and which reduced? Which have lost energy and which have gained energy? What process allows some organisms to do anaerobic cellular respiration for a short period of time? Describe fermentation. What happens and why is it important? Which molecule is oxidized, which is reduced? Describe the structure of mitochondria and tell what happens in each area. List 3 reactants that go into the Kreb’s (TCA or citric acid) cycle and 3 products that come out. Which have been oxidized and which reduced? Which have lost energy and which have gained energy? What two processes combine to form the oxidative phosphorylation pathway? List 3 reactants that go into oxidative phosphorylation and 3 products that come out. Which have been oxidized and which reduced? Which have lost energy and which have gained energy? What is chemiosmosis and what structure is used to do this? Why is chemiosmosis important? What directly powers chemiosmosis? What role do the electron transport chain (ETC) enzymes play in this process? What is the electron donor (gets oxidized) and the electron acceptor (gets reduced) in cellular respiration? What is the primary reason that living organisms do cellular respiration? Write the summary equation for photosynthesis. Where within the plant and algae cells does photosynthesis occur? Where does the energy come from to power photosynthesis? Overall, is photosynthesis exergonic or endergonic, catabolic or anabolic, oxidation or reduction? Describe the nature of light (electromagnetic) energy. Is light kinetic or potential energy? What part of the electromagnetic spectrum powers photosynthesis? What role do accessory pigments play? Describe photosystems. Where are they located, what do they do and what do they produce? What is the difference between photosystem I and photosystem II? Which is necessary for photosynthesis? Describe the structure of chloroplasts. Tell where each stage of photosynthesis occurs. List the 3 steps, or stages, in the light reactions of photosynthesis. Follow an electron through both photosystems. How is chemiosmosis related to the photosystems? List 3 reactants that go into the light reactions (+ energy) and 3 products that come out. What form of energy goes into the light reactions and what form of energy comes out? Which molecule is oxidized and which molecule is reduced in the light reactions of photosynthesis? What is the importance of chemiosmosis in photosynthesis? Overall, what happens in the Calvin cycle (dark reactions) of photosynthesis and where does it occur? List 3 reactants that go into the dark reactions and 3 products that come out. Which molecule is oxidized and which molecule is reduced in the dark reactions? Overall in photosynthesis, which molecule is oxidized (electron donor) and which is reduced (electron acceptor)? Which form of energy goes in and which form of energy comes out? Define photorespiration and tell how it is related to environmental factors that regulate photosynthesis. Describe two ways that some plants have evolved to avoid photorespiration. List the key events, in order, as biologists think they occurred in the evolution of metabolic diversity. Describe the endosymbiotic hypothesis. What cell organelles do we think originated this way?