* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Metabolism of drugs
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Compounding wikipedia , lookup
Plateau principle wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Specialty drugs in the United States wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Drug design wikipedia , lookup
Orphan drug wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
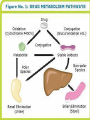
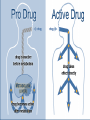
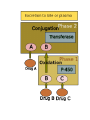
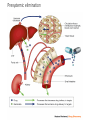
Drug Biotransformation Elimination of the drugs Drug Biotransformation Metabolism or biotransformation complex of processes which provide decreasing of toxicity and accelerate excreting of the molecule of a drug or other foreign substance after its incoming into the organism (Chemical alteration of the drug in the body ) Metabolism of Drugs • Aim: to convert non-polar lipid soluble compounds to polar lipid insoluble compounds to avoid reabsorption in renal tubules • Most hydrophilic drugs are less biotransformed and excreted unchanged – streptomycin, neostigmine and pancuronium etc. • Biotransformation is required for protection of body from toxic metabolites Results of Biotransformation • Active drug and its metabolite to inactive metabolites – most drugs (ibuprofen, paracetamol, chlormphenicol etc.) • Active drug to active product (phenacetin – acetminophen or paracetamol, morphine to morphine-6-glucoronide, digitoxin to digoxin etc.) • Inactive drug to active/enhanced activity (prodrug) – levodopa - carbidopa, prednisone – prednisolone and enalapril – enalaprilat) • No toxic or less toxic drug to toxic metabolites (Isonizide to Acetyl isoniazide) Biotransformation of drugs into active (or more active) metabolites Initial drug • Allopurinol • Amitriptilin • Acetylsalicylic acid • Butadion • Diazepam • Digitoxin • Codein • Cortizol • Methyldopa • Prednison • Novocainamid • Propranolol Active metabolite • Aloxantin • Nortriptilin • Salicylic acid • Oxyfenbutazon • Dismethyldiazepam • Digoxin • Morphine • Hydrocortizon • Methylnoradrenalin • Prednisolon • N-acetylnovocainamid • N-oxypropranolol ORGANS OF DRUGS METABOLISM •liver • kidneys • muscle tissue • intestinal wall • lungs • skin • blood Reactions of biotransformation • Nonsynthetic - І phase – metabolite may be active or inactive • Synthetic - ІІ phase – metabolites are inactive (Morphine – M-6 glucoronide is exception) І phase (nonsynthetic reactions): (oxydation, reduction, hydrolysis) • 1) microsomal reactions • 2) nonmicrosomal reactions Reactions of І phase - transformation in molecule with formation of functional groups with active hydrogen atom Phase I - Oxidation • Most important drug metabolizing reaction – addition of oxygen or (–ve) charged radical or removal of hydrogen or (+ve) charged radical • Various oxidation reactions are – oxygenation or hydroxylation of C-, N- or S-atoms; N or 0dealkylation • Examples – Barbiturates, phenothiazines, paracetamol and steroids Phase I - Oxidation • Involve – cytochrome P-450 monooxygenases (CYP), NADPH and Oxygen • More than 100 cytochrome P-450 isoenzymes are identified and grouped into more than 20 families – 1, 2 and 3 … • Sub-families are identified as A, B, and C etc. • In human - only 3 isoenzyme families important – CYP1, CYP2 and CYP3 • CYP 3A4/5 carry out biotransformation of largest number (30– 50%) of drugs. In addition to liver, this isoforms are expressed in intestine (responsible for first pass metabolism at this site) and kidney too • Inhibition of CYP 3A4 by erythromycin, clarithromycin, ketoconzole, itraconazole, verapamil, diltiazem and a constituent of grape fruit juice is responsible for unwanted interaction with terfenadine and astemizole • Rifampicin, phenytoin, carbmazepine, phenobarbital are inducers of the CYP 3A4 The catalytic cycle of cytochrome P450 CYP-450 – hemoprotein, which is able to interact with substrate of oxydation, to activate oxygen and combine it with substrate. Specifically on CYР-450 reactions of hydroxydation are performed large amount of isoforms of this enzyme – possibility of its binding with different substrates and taking part in their metabolism There are 24 isoforms of CYР-450 in microsomes of human liver Multiplicity of the enzyme has a group character: one isoform of CYР-450 interacts not only with one substrate but with a group of substances Microsomal enzyme system Oxydoreductases, esterases, enzymes of proteins, lipids, glycerophosphatides, lipo- and glycoproteids, bile acids, cholesterol, prostaglandins biosynthesis, enzyme systems of biosynthesis of couple compounds, ethers of glucuronic and sulfur acids Oxydoreductases of microsomes (oxygenases of microsomes, microsomal hydroxydating system, NADPH-hydroxylase system, monooxygenases of mixed functions) – these are enzymes which activate molecular oxygen and catalize including of one (monooxygenase) or two (dioxygenases) atoms of oxygen into molecule of substrate (R) Reaction is presented as follows: R + O2 + DН = ROH + H2O + D One atom of О2 is included into molecule of the substrate, other is reduced to Н2О, therefore enzyme performs oxygenase and oxydase functions simultaneously. That’s why monooxygenases ate also called oxydases of mixed function. Along with this hydroxyl group (-ОН) forms in molecule of substrate, that’s why monooxygenase is also calles hydroxylating system, and reaction of oxydation – oxydating hydroxylation Nonmicrosomal Enzyme Oxidation • Some Drugs are oxidized by non-microsomal enzymes (mitochondrial and cytoplsmic) – Alcohol, Adrenaline, Mercaptopurine • Alcohol – Dehydrogenase • Adrenaline – MAO • Mercaptopurine – Xanthine oxidase Phase I - Reduction • This reaction is conversed of oxidation and involves CYP 450 enzymes working in the opposite direction. • Examples - Chloramphenicol, levodopa, halothane and warfarin • Levodopa (DOPA) Dopamine DOPA-decarboxylase Phase I - Hydrolysis • This is cleavage of drug molecule by taking up of a molecule of water. Similarly amides and polypeptides are hydrolyzed by amidase and peptidases. Hydrolysis occurs in liver, intestines, plasma and other tissues. • Examples - Choline esters, procaine, lidocaine, pethidine, oxytocin 1- Esterase enzymes: • Usually present in plasma and various tissues, are nonspecific and catalyze deesterification. Hydrolysis of nonpolar esters into two polar and more water soluble compounds (i.e. acid and alcohol). O O CH3 CH3 C OR + H2O Ester of acetic acid C OH + ROH Acetic acid Alcohol Esterases are responsible for converting many prodrugs into their active forms. A classical example of ester hydrolysis is the metabolic conversion of aspirin (acetylsalicylic acid) to salicylic acid and COOH acetic acid. COOH OCOCH3 H2O OH + CH3COOH Acetic acid Aspirin Salicylic acid 2-Amidase enzymes: It is the hydrolysis of amides into amine and acid and this is called Deamination.Deamination occurs primarily in the liver. O O NH2 C R Amide + H2O Water R C OH + NH3 Acid Ammonia •Amide drugs are more resistant to hydrolysis (or they are not hydrolyzed until they reach the liver) than ester drugs which they are susceptible to plasma esterase. •The duration of actions of ester drugs are less than the amide analogues. Example: Procaine (ester type) injection or topical is usually shorter acting than its amide analogue procainamide administered similarily. Phase II metabolism • Conjugation of the drug or its phase I metabolite with an endogenous substrate - polar highly ionized organic acid to be excreted in urine or bile - high energy requirements • Glucoronide conjugation - most important synthetic reaction • Compounds with hydroxyl or carboxylic acid group are easily conjugated with glucoronic acid - derived from glucose • Examples: Chloramphenicol, aspirin, morphine, metroniazole, bilirubin, thyroxine • Drug glucuronides, excreted in bile, can be hydrolyzed in the gut by bacteria, producing beta-glucoronidase - liberated drug is reabsorbed and undergoes the same fate - enterohepatic recirculation (e.g. chloramphenicol, phenolphthalein, oral contraceptives) and prolongs their action Phase II metabolism – contd. • Acetylation: Compounds having amino or hydrazine residues are conjugated with the help of acetyl CoA, e.g.sulfonamides, isoniazid • Genetic polymorphism (slow and fast acetylators) • Sulfate conjugation: The phenolic compounds and steroids are sulfated by sulfokinases, e.g. chloramphenicol, adrenal and sex steroids Phase II metabolism – contd. • Methylation: The amines and phenols can be methylated. Methionine and cysteine act as methyl donors. • Examples: adrenaline, histamine, nicotinic acid. • Ribonucleoside/nucleotide synthesis : activation of many purine and pyrimidine antimetabolites used in cancer chemotherapy Main ways of biotransformation of drugs • I phase • Oxydation: diazepam, pentazocin, sydnocarb, phenotiazin, phenobarbital, aspirin, butadion, lidokain, morphin, codein, ethanol, rifampicin • Reduction: hestagens, metronidazol, nitrazepam, levomycetin, chlozepid • Hydrolysis: levomycetin, novocain, cocain, glycosides, ditilin, novocainamid, xycain, fentanyl • II phase • • • • • Conjugation with sulfate: morphin, paracetamol, isadrin Conjugation with glucuronic acid: teturam, sulfonamides, levomycetin, morphin Conjugation with remains of - aminoacids: nicotinic acid, paracetamol Acetylation: sulfonamides, isoniasid, novocainamid Methylation: morphin, unitiol, ethionamid, noradrenalin Metabolism in the intestinal wall Synthetic and nonsynthetic reactions take place • Isadrin – conjugation with sulfate • Hydrlalasin - acetylation • Penicillin, aminazin – metabolism with nonspecific enzymes • Methotrexat, levodopa – metabolism with intestinal bacteria Factors affecting Biotransformation Concurrent use of drugs: Induction and inhibition • Genetic polymorphism • Pollutant exposure from environment or industry • Pathological status • Age Factors that influence on drug metabolism Factor Reaction type Age (newborns, Decreasing of metabolism speed children, elderly) Pregnancy Increasing of metabolism speed Genetic factor Various reactions Liver pathology Decreasing of excreation speed of drugs, depending on their kinetics, type and stage of liver disease, increasing of bioavailability and decreasing of excretion speed of orally administered drugs with high hepatic clearence GI pathology Changes in metabolism in GI epithelium Nutrition character Increasing of metabolism speed of certain drugs in case of diet with dominance of proteins and carbohydrates Decreasing of metabolism speed in case of heavy digestive disorders linked with starvation (total or protein) Environment Alcohol — one time consumption — chronic consumption Smoking Way of excretion Increasing of metabolism speed if in contact with chlorine insecticides Depressing of enzymes that metabolise drugs Induction of enzyme system Increasing of metabolism of certain drugs (i.e. theophyllin) Metabolism in liver before entering system circulation (first going-through effect) after peroral administration of drugs Circade changes in drugs metabolism Time of introduction of drugs Interaction of Stimulation drugs reaction and depression of enzyme USING DRUGS DURING LACTATION Absolutely contraindicated • Antibacterial: tetracyclins, levomycetin, fluoroquinolones, sulfonamides, nalidixic acid, metronidazole • Antiviral: amantadin, gancyclovir, zidovudin, remantadin • Cytostatics • Drugs effecting CNS: difenin, sodium valproate, lithium preparations, barbiturates, reserpin, opioid analgesics (regularly) • Drugs of other groups: iodides, antithyroid drugs, undirect anticoagulants, radiopharmaceutical drugs (radioactive iodine etc.), Ergot alkaloids, chlorpropramid, cyclosporin USING DRUGS DURING LACTATION (continuation) Undesirable Bromides, meprobamat, derivatives of benzodiazepine (diazepam, chlozepid, oxazepam etc.), aminazin, ethosuximid; M-cholinoblockers, glucocorticosteroids (if dosage is over 100 mg per day), indometacin, salycylates (large doses), derivatives of sulfonilurea, theophyllin, chloroquin, nitrofuran derivatives (furazolidon etc.), isoniazid, cymetidin, aluminum containing antacids, estrogens, gold medications, retinoids Enzyme Inhibition • One drug can inhibit metabolism of other – if utilizes same enzyme • However not common because different drugs are substrate of different CYPs • A drug may inhibit one isoenzyme while being substrate of other isoenzyme – quinidine • Some enzyme inhibitors – Omeprazole, metronidazole, isoniazide, ciprofloxacin and sulfonamides Microsomal Enzyme Induction • CYP3A – antiepileptic agents - Phenobarbitone, Rifampicin and glucocorticoide • CYP2E1 - isoniazid, acetone, chronic use of alcohol • Other inducers – cigarette smoking, charcoal broiled meat, industrial pollutants – CYP1A • Consequences of Induction: • Decreased intensity – Failure of OCPs • Increased intensity – Paracetamol poisoning (NABQI) • Tolerance – Carbmazepine • Some endogenous substrates are metabolized faster – steroids, bilirubin Influence of body weight on kinetics of drugs • In exhausted patients – speeding up of elimination, that’s why it’ s appropriate to introduce the increased dose – 1+1/3 • In patients with overweighting – retention of lipid-soluble drugs in the organism • For these patients it’s suitable to correct the dose according to “ideal” body weight: For men ІBW = 50 + [(Н - 150) : 2,5] For women ІBW = 45 + [(Н - 150) : 2,5] where Н – height in cm • in case of normal body weight the dose is calculated counting on 1 kg of patient’s body weight Drug-Drug Interactions during Metabolism Many substrates are retained not only at the active site of the enzyme but remain nonspecifically bound to the lipid membrane of the endoplasmic reticulum. In this state, they may induce microsomal enzymes; depending on the residual drug levels at the active site, they also may competitively inhibit metabolism of a simultaneously administered drug. Drug-Drug Interactions during Metabolism Enzyme-inducing drugs include various sedative-hypnotics, tranquilizers, anticonvulsants, and insecticides. Patients who routinely ingest barbiturates, other sedative-hypnotics, or tranquilizers may require considerably higher doses of warfarin (an oral anticoagulant) to maintain a prolonged prothrombin time. On the other hand, discontinuance of the sedative may result in reduced metabolism of the anticoagulant and bleeding—a toxic effect of the ensuing enhanced plasma levels of the anticoagulant. Similar interactions have been observed in individuals receiving various combination drug regimens such as antipsychotics or sedatives with contraceptive agents, sedatives with anticonvulsant drugs, and even alcohol with hypoglycemic drugs (tolbutamide). Drug-Drug Interactions during Metabolism Simultaneous administration of two or more drugs may result in impaired elimination of the more slowly metabolized drug and prolongation or potentiation of its pharmacologic effects Both competitive substrate inhibition and irreversible substrate-mediated enzyme inactivation may augment plasma drug levels and lead to toxic effects from drugs with narrow therapeutic indices. Drug-Drug Interactions during Metabolism Allopurinol both prolongs the duration and enhances the chemotherapeutic action of mercaptopurine by competitive inhibition of xanthine oxidase. Consequently, to avoid bone marrow toxicity, the dose of mercaptopurine is usually reduced in patients receiving allopurinol. Cimetidine, a drug used in the treatment of peptic ulcer, has been shown to potentiate the pharmacologic actions of anticoagulants and sedatives. The metabolism of the sedative chlordiazepoxide has been shown to be inhibited by 63% after a single dose of cimetidine; such effects are reversed within 48 hours after withdrawal of cimetidine. PRESYSTEMIC ELIMINATION Presystemic elimination – extraction of the drug from blood circulatory system during it’s first going through the liver (first pass metabolism) – it leads to decreasing of bioavailability (and therefore, decreasing of biological activity) of drugs propranolol (anaprilin), labetolol, aminazin, acetylsalicylic acid, labetolol, hydralasin, isadrin, cortizone, lidokain, morphin, pentasocin, organic nitrates, reserpin Presystemic elimination Clinical Relevance of Drug Metabolism The dose and the frequency of administration required to achieve effective therapeutic blood and tissue levels vary in different patients because of individual differences in drug distribution and rates of drug metabolism and elimination. These differences are determined by genetic factors and nongenetic variables such as age, sex, liver size, liver function, circadian rhythm, body temperature, and nutritional and environmental factors such as concomitant exposure to inducers or inhibitors of drug metabolism. Elimination of the drugs drugs can be excreted in forms of metabolites or unchanged forms kidneys, liver, lungs, intestines, sweat and mammary glands etc. through different ways: Hydrophilic compounds can be easily excreted. Elimination through kidneys filtration, canalicular secretion and canalicular reabsorption • filtration (relative molecular weight of drugs is less than 90, if 90-300 – with urine and bile): ampicillin, gentamicin, urosulfan, novokainamid, digoxin • Disorders of filtration – shock, collapse (due to decreasing of blood circulation and hydrostatic pressure of blood plasma in glomerular capillaries) • furosemide (closely connected with plamsa proteins) is not filtrated in glomerular capilaries • canalicular secretion – active process (with the aid of enzyme system and using energy): penicillins, furosemide, salicilates, chinin • Disorders of canalicular secretion – in case of disorders of energetic metabolism in kidneys: hypoxia, infections, intoxications Glomerular Filtration • Normal GFR – 120 ml/min • Glomerular capillaries have pores larger than usual • The kidney is responsible for excreting of all water soluble substances • All nonprotein bound drugs (lipid soluble or insoluble) presented to the glomerulus are filtered • Glomerular filtration of drugs depends on their plasma protein binding and renal blood flow - Protein bound drugs are not filtered ! • Renal failure and aged persons Tubular Re-absorption • Back diffusion of Drugs (99%) – lipid soluble drugs • Depends on pH of urine, ionization etc. • Lipid insoluble ionized drugs excreted as it is – aminoglycoside (amikacin, gentamicin, tobramycin) • Changes in urinary pH can change the excretion pattern of drugs • Weak bases ionize more and are less reabsorbed in acidic urine. • Weak acids ionized more and are less reabsorbed in alkaline urine • Utilized clinically in salicylate and barbiturate poisoning – alkanized urine (Drugs with pKa: 5 – 8) • Acidified urine – atropine and morphine etc. Tubular reabsorbtion (reversed absorbtion) lipid-soluble drugs are reabsorbed passively ionized drugs, which are weak acids or alkali are reabsorbed actively regulation of level of reabsorbtion - to speed up elimination of drugs – weak alkalis (antihistamine drugs, chinin, theophyllin) urine is made acidic (with ascorbinic acid, ammonium chloride) - to speed up elimination of drugs – weak acids (NSAID, including ASA, barbiturates, sulfonamides) urine is made alkaline (introduction of sodium hydrocarbonate) Tubular Secretion • Energy dependent active transport – reduces the free concentration of drugs – further, more drug dissociation from plasma binding – again more secretion (protein binding is facilitatory for excretion for some drugs) ELIMINATION OF DRUGS (cont’d) with bile – drugs and their metabolites with relative MM over 3000 enterohepatic (intestinal-liver) recirculation: cardiac glycosides, morphine, tetracyclines are excreted with bile in unchanged condition (previously not metabolized): antibiotics of tetracyclines group, macrolides through lungs – gases and volatile substances: ether for narcosis, ftorotan, N2O, partly – camphor, iodides, ethanol through intestine: ftalasol, enteroseptol, magnesium sulfate through sweat glands: iodides, bromides, salicylates through bronchial, salivary glands: bromides, iodides with milk: get into organism of the baby – levomycetin, fenilin, reserpin, lithium remedies, meprotan, tetracyclines, sulfonamides etc. THANK YOU -PHARMA STREET