* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapters 1 and 2
Vapor-compression refrigeration wikipedia , lookup
Space Shuttle thermal protection system wikipedia , lookup
Thermal comfort wikipedia , lookup
Underfloor heating wikipedia , lookup
Insulated glazing wikipedia , lookup
Passive solar building design wikipedia , lookup
Solar water heating wikipedia , lookup
Building insulation materials wikipedia , lookup
Dynamic insulation wikipedia , lookup
Heat exchanger wikipedia , lookup
Thermal conductivity wikipedia , lookup
Intercooler wikipedia , lookup
Solar air conditioning wikipedia , lookup
Cogeneration wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat equation wikipedia , lookup
R-value (insulation) wikipedia , lookup
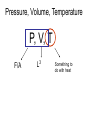
"Experiment is the interpreter of nature. Experiments never deceive. It is our judgment which sometimes deceives itself because it expects results which experiment refuses." - Leonardo da Vinci (1452-1519) Thermodynamics • Large number of particles ~ N = 6.022 1023 = mole (the quantity of matter that contains as many identical objects [e.g. atoms, molecules, formula units, ions] as the number of atoms in exactly 12 g of 12C) A • Fluctuations are small and can often be ignored, i.e., the thermo-dynamic limit F d (mv ) / dt Thermodynamic limit Pressure, Volume, Temperature P, V, T F/A L3 Something to do with heat What is heat ? Heat is energy in transit ! Surroundings System Heat Universe = (system + surroundings) Heat is measured in Joules What is temperature? Temperature is what you measure with a thermometer Temperature is the thing that’s the same for two objects, after they’ve been in contact long enough. Long enough so that the two objects are in thermal equilibrium. Time required to reach thermal equilibrium is the relaxation time. Temperature is usually measured in K, C or F and cannot be expressed in mechanical units (mass, length and time). Temperature is a measure of the tendency of an object to spontaneously give up/absorb energy to/from its surroundings. Diathermal wall is a system boundary that freely allows heat to be exchanged, i.e. very short relaxation time for systems separated by a diathermal wall. Adiabatic wall is a system boundary that does not allow heat to be exchanged, i.e. very long relaxation time for systems separated by a adiabaric wall. T 1- T 2 T 1- T 2 Adiabatic wall time time VG-8 Ideal gas equation of state Boyle’s law p 1/V Robert Boyle (1627 – 1691) Charles’ law pV = NkB T VT Jacques Charles (1746 – 1823) Gay-Lussac’ law kB = 1.38 10-23 J/K pT Joseph Louis Gay-Lussac (1778 - 1850) VG - 9 How much “energy in transit” needed to raise temperature of an object by dT ? Heat capacity (units JK -1) dQ C dT Q CV T V Q CP T P Specific heat capacity (c) is the heat capacity per unit mass. Specific heat of a solid Copper Measurement of heat capacity by relaxation calorimetry Thermometer needs to have a much lower heat capacity than the sample T T0 + T T0 + T T0 T0 Heater Sample Thermometer t