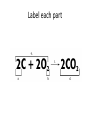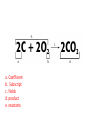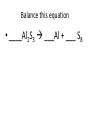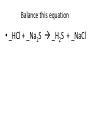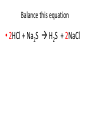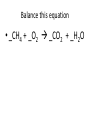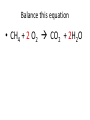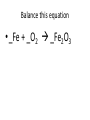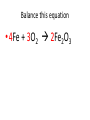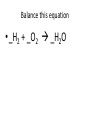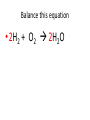* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch. 9 Test Review
California Green Chemistry Initiative wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
History of chemistry wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical Corps wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical plant wikipedia , lookup
Double layer forces wikipedia , lookup
Safety data sheet wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Chemical potential wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Electrochemistry wikipedia , lookup
Process chemistry wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Chemical reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Click chemistry wikipedia , lookup
Rate equation wikipedia , lookup
Detailed balance wikipedia , lookup
George S. Hammond wikipedia , lookup
VX (nerve agent) wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Ch. 9 Test Review the process by which one or more substances change to produce one or more different substances Chemical reaction a solid that is produced as a result of a chemical reaction in solution precipitate a chemical reaction in which heat is released to the surroundings Exothermic reaction the law that states that energy cannot be created or destroyed but can be changed from one form to another Law of conservation of Energy A chemical reaction that requires heat Endothermic reaction a combination of chemical symbols and numbers to represent a substance Chemical Formula a substance that forms in a chemical reaction Product a representation of a chemical reaction that uses symbols to show the relationship between the reactants and products Chemical Equation the law that states that mass cannot be created or destroyed in an ordinary physical and chemical change Law of conservation of Mass a substance or molecule that participates in a chemical reaction Reactant Label each graph Energy Released Activation Energy Activation Energy Label each graph Energy Absorbed Endothermic Reaction Exothermic Reaction Label each part a. Coefficient b. Subscript c. Yields d. product e. reactants List the 8 Diatomic Elements A. B. C. D. E. F. G. H. List the 8 Diatomic Elements A. Hydrogen B. Nitrogen C. Oxygen D. Fluorine E. Chlorine F. Bromine G. Iodine H. Astatine Balance this equation • ____Al2S3 ___Al + ___ S8 Balance this equation • 8 Al2S3 16 Al + 3 S8 Balance this equation • _HCl + _Na2S _H2S + _NaCl Balance this equation • 2HCl + Na2S H2S + 2NaCl Balance this equation • _CH4 + _O2 _CO2 + _H2O Balance this equation • CH4 + 2 O2 CO2 + 2H2O Balance this equation •_Fe + _O2 _Fe2O3 Balance this equation •4Fe + 3O2 2Fe2O3 Balance this equation •_H2 + _O2 _H2O Balance this equation •2H2 + O2 2H2O