* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Discussion Sheet 11
Marcus theory wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Discodermolide wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Ene reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Elias James Corey wikipedia , lookup
Stille reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
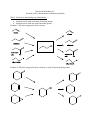
Discussion Worksheet #11 Alcohols As Key Intermediates in Multistep Synthesis Skill 1: Alcohols as functional group intermediates Alcohols can be made from many functional groups Alcohols can be made into many functional groups Problem 1. Fill in the reagents for these reactions Problem 2. Fill in the reagents for these reactions, as well as functional group names. Problem 3. Use problems 1 and 2 as a flow chart to propose two step transformations with alcohols as synthetic intermediates.: Skill 2: Alcohols can be made into leaving groups Alcohols can be made into a variety of leaving groups This opens up possibilities for Sn2 and E2 reactions. Problem 4: Elimination of alcohols can be performed with POCl3 or concentrated sulfuric acid, but these are sometimes limited. Explain why the following reaction won’t work, and propose an alternative. Problem 5. Explain why the following substitution reaction will not work, and provide an alternative. Skill 3: Reactions of alcohols are key C-C bond forming steps in multistep synthesis Oxidation of alcohols produce electrophiles that can be used in a future C-C bond forming step Transformation of an alcohol into an alkylhalide, followed by Grignard formation, allows an alcohol to be made into a nucleophile Problem 6: Is an electrophile or nucleophile needed in each reaction? Provide the necessary reagents. Skill 4: Protecting groups Alcohols are week acids. If a strong base is used in a reaction, the alcohol must be protected. Silyl ethers are good options to protect alcohols because they are unreactive under basic conditions, but then can be removed easily. Problem 7. Use a protecting group in each of these syntheses. Explain why it is necessary in each case.