* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 15
Survey
Document related concepts
Cell membrane wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell nucleus wikipedia , lookup
Endomembrane system wikipedia , lookup
Extracellular matrix wikipedia , lookup
Signal transduction wikipedia , lookup
Programmed cell death wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Spindle checkpoint wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Transcript
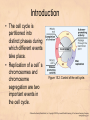
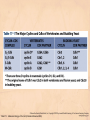
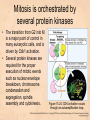
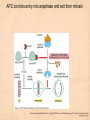
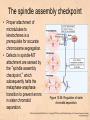
Chapter 15 Cell Cycle Regulation Introduction • A cell contains all the information necessary for making a copy of itself during a cell division cycle. • The eukaryotic cell division cycle (cell cycle) is composed of an ordered set of events that results in the generation of two copies of a preexisting cell. Figure 15.1: Stages in a typical mammalian cell cycle. MPF is a dimer of a mitotic cyclin and cyclin-dependent kinase (cdk) Cyclin B levels and MPF activity change together in cycling Xenopus egg extracts Figure 13-7 Introduction • The cell cycle is partitioned into distinct phases during which different events take place. • Replication of a cell’s chromosomes and chromosome segregation are two important events in the cell cycle. Figure 15.2: Control of the cell cycle. The Cell Cycle (5) • The Role of Protein Kinases – Entry into the M phase is triggered by activation of a protein kinase called maturation promoting factor (MPF). • MPF consists of two subunits: a kinase and a regulatory subunit, cyclin. • Increased concentration of cyclin activates the kinase. • The cyclin levels fluctuate predictably during the cell cycle. Fluctuations of cyclin and MPF levels during the cell cycle The Cell Cycle (7) • Cyclin Binding – Cyclin binds to the catalytic subunit of Cdk. – Different cyclins are transcribed at different points in the cell cycle. – Cyclin-Cdk complexes phosphorylate other proteins. Components of the cell cycle control system *The control of cell cycle regulation; a clock or timer; specific time -correct order-once per cycle -binary switch on/off system -adaptability; specific cell types or environmental •Checkpoint control; delay the cell cycle progression or arrest the cell cycle in response to signals -send a negative signal rather than removal of positive signal •-provide time for DNA repair or prevent the disaster Table 17-1 Molecular Biology of the Cell (© Garland Science 2008) A cycle of cyclin-dependent kinase activities regulate cell proliferation • Cyclin-dependent Kinases (CDKs) are master regulators of the cell cycle. • CDKs are active only when complexed with a cyclin protein. Figure 15.11: CDK-cyclin complexity in fission yeast and mammalian cells. A cycle of cyclin-dependent kinase activities regulate cell proliferation • Cyclins are proteins that oscillate in abundance during the cell cycle. • A CDK partners with different cyclins during different phases of the cell cycle to phosphorylate distinct sets of target proteins. Figure 15.12: The level of Cdk1-cyclin B activity. CDK-cyclin complexes are regulated in several ways • CDK-cyclin complexes are regulated by phosphorylation, inhibitory proteins, proteolysis, and by subcellular localization. Figure 15.13: Regulation of Cdk1 by phosphorylation. Cells may withdraw from the cell cycle • Cell divisions are controlled by external stimuli and nutrient availability, and are not continuous. • Cells can be in a nonproliferating state called quiescence, or G0. • Cells reenter the cell cycle primarily in G1. • Cells can permanently leave the cell cycle, becoming senescent. Entry into cell cycle is tightly regulated • Cells detect the presence of chemical signals in their environment using a variety of cell surface receptors. • Extracellular signals elicit intracellular biochemical responses that can impinge on the activity of the cell cycle control engine. • Some extracellular signals induce cells to self-destruct by apoptosis. DNA replication requires assembly of protein complexes • Replication occurs after cells progress through the restriction point or START. • Replication is regulated in a stepwise fashion and is coordinated with the completion of mitosis. Figure 15.20: Assembly of the prereplication complex. DNA replication requires assembly of protein complexes • Replication occurs at origins that may be defined by sequence, by position, or by spacing mechanisms. • Initiation occurs only at origins that are licensed to replicate. • Once fired, origins cannot be reused until the next Figure 15.22: cell cycle. Initiation of DNA synthesis. Mitosis is orchestrated by several protein kinases • The transition from G2 into M is a major point of control in many eukaryotic cells, and is driven by Cdk1 activation. • Several protein kinases are required for the proper execution of mitotic events such as nuclear envelope breakdown, chromosome condensation and segregation, spindle assembly and cytokinesis. Figure 15.24: CDK activation occurs through an autoamplification loop. Sister chromatids are held together until anaphase • A mitotic chromosome consists of two sister chromatids held together by cohesive forces. • Ubiquitin-mediated proteolysis drives the release of sister chromatid cohesion and marks the onset of anaphase. • The anaphase promoting complex or cyclosome (APC/C) is the E3 ubiquitin ligase that directs proteolysis of key mitotic proteins including securin and cyclin. Sister chromatids are held together until anaphase Figure 15.26: Regulation of sister chromatid separation. APC controls entry into anaphase and exit from mitosis Exit from mitosis requires more than cyclin proteolysis • Exit from mitosis requires Cdk1 inactivation. • Mitotic exit also involves the reversal of Cdk1 phosphorylation. Figure 15.27: Temporal and substrate specificity of the APC. Events of the cell cycle are coordinated • Processes in the cell cycle occur in an irreversible order. • The major events of the cell cycle occur once and only once in each round of cell division. • Checkpoints ensure the error-free completion of one cell cycle event before the next process begins. Figure 15.30: The cell cycle has many checkpoints. Checkpoints in cell cycle regulation Figure 13-34 Checkpoint controls coordinate different cell cycle events • Cell cycle events are coordinated with one another. • The coordination of cell cycle events is achieved by the action of specific biochemical pathways called checkpoints that delay cell cycle progression if a previous cell cycle event is not completed. • Checkpoints may be essential only when cells are stressed or damaged but may also act during a normal cell cycle to ensure proper coordination of events. DNA replication and DNA damage checkpoints monitor defects in DNA metabolism • Incomplete and/or defective DNA replication activates a cell cycle checkpoint. • Damaged DNA activates a different checkpoint that shares some components with the replication checkpoint. • The DNA damage checkpoint halts the cell cycle at different stages depending on the stage during which the damage occurred. DNA replication and DNA damage checkpoints Figure 15.34: Regulation of G1-S transition by the DNA damage checkpoint. The spindle assembly checkpoint monitors defects in chromosomemicrotubule attachment. • The mitotic spindle attaches to individual kinetochores of chromosomes during mitosis. Figure 15.37: Types of chromomsome attachment. The spindle assembly checkpoint • Proper attachment of microtubules to kinetochores is a prerequisite for accurate chromosome segregation. • Defects in spindle-MT attachment are sensed by the “spindle assembly checkpoint,” which subsequently halts the metaphase-anaphase transition to prevent errors in sister chromatid separation. Figure 15.38: Regulation of sister chromatid separation. Cell cycle deregulation can lead to cancer • Proto-oncogenes encode proteins that drive cells into the cell cycle. • Tumor-suppressor genes encode proteins that restrain cell cycle events. • Mutations in proto-oncogenes, tumor suppressor genes, or checkpoint genes may lead to cancer.