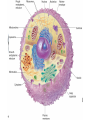
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Slides for Chapter 1-4 - Department of Chemistry and Physics
Cracking (chemistry) wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
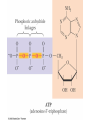
Discodermolide wikipedia , lookup
Kinetic isotope effect wikipedia , lookup
Elias James Corey wikipedia , lookup
Marcus theory wikipedia , lookup
Aldol reaction wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Stille reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Organic Chemistry Supplement Alkyl Halides React with Nucleophiles and Bases Alkyl halides are polarized at the carbon-halide bond, making the carbon electrophilic Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce elimination 2 11.2 The SN2 Reaction Reaction is with inversion at reacting center Follows second order reaction kinetics Ingold nomenclature to describe characteristic step: S=substitution N (subscript) = nucleophilic 2 = both nucleophile and substrate in characteristic step (bimolecular) 3 SN2 Transition State The transition state of an SN2 reaction has a planar arrangement of the carbon atom and the remaining three groups 4 Stereochemistry of SN1 Reaction The planar intermediate leads to loss of chirality A free carbocation is achiral Product is racemic or has some inversion 5 11.6 Biological Substitution Reactions SN1 and SN2 reactions are well known in biological chemistry Unlike what happens in the laboratory, substrate in biological substitutions is often organodiphosphate rather than an alkyl halide 6 11.8 The E2 Reaction and the Deuterium Isotope Effect A proton is transferred to base as leaving group begins to depart Transition state combines leaving of X and transfer of H Product alkene forms stereospecifically 7 3 This can be extended to Phosphoanhydride bonds found in ATP 5