* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Week 10 Day 2-Plastics - Washington State University
Survey
Document related concepts
Pseudo Jahn–Teller effect wikipedia , lookup
Glass transition wikipedia , lookup
Crystal structure wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Path integrals in polymer science wikipedia , lookup
Molecular nanotechnology wikipedia , lookup
Viscoelasticity wikipedia , lookup
Self-healing hydrogels wikipedia , lookup
Strengthening mechanisms of materials wikipedia , lookup
Sol–gel process wikipedia , lookup
Transcript
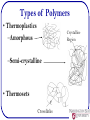
Polymers: what they are and how they work? Michael R. Kessler School of Mechanical and Materials Engineering Washington State University What are Polymers • Examples of polymers and where they are used. • Often Synthetic Polymers are called Plastic. • The volume of polymers produced is three times larger than that of all metals! • Poly (many) mer (parts) made up of molecules of very long chains. What is a Polymer? • Poly (many) mer (parts) made up of molecules of very long chains. repeat unit repeat unit H H H H H H C C C C C C H H H H H H H H H H H H C C C C C C H Cl H Cl H Cl Polyethylene (PE) repeat unit H C H Poly(vinyl chloride) (PVC) H H C C CH3 H H H C C CH3 H H C CH3 Polypropylene (PP) Ancient Polymers • Originally natural polymers were used – Wood – Cotton – Leather – Rubber – Wool – Silk • Oldest known uses – Rubber balls used by Incas – Noah used pitch (a natural polymer) for the ark Polymer Composition Most polymers are hydrocarbons – i.e., made up of H and C • Saturated hydrocarbons – Each carbon singly bonded to four other atoms – Example: » Ethane, C2H6 H H C H H C H H Chemistry and Structure of Polyethylene Note: polyethylene is a long-chain hydrocarbon - paraffin wax for candles is short polyethylene Bulk or Commodity Polymers Bulk or Commodity Polymers (cont) Bulk or Commodity Polymers (cont) Analogy for the Structure of Polymers Properties of Polymers • Glass transition temperature (snake pit analogy) • Strength—measured in maximum stress (force per unit area) • Stiffness—measured in stress per unit strain • Damping—ability to absorb energy Properties are often temperature and rate dependent (Viscoelastic) Properties (Demonstration) Happy Ball Sad Ball •Compare the damping properties for two apparently identical polymer balls by bouncing them on the floor. •How do their damping behaviors vary with temperature? Happy Ball = Neoprene (polychloroprene) Sad Ball = Norsorex (polynorbornene) MOLECULAR WEIGHT • Molecular weight, M: Mass of a mole of chains. Low M high M Not all chains in a polymer are of the same length — i.e., there is a distribution of molecular weights MOLECULAR WEIGHT DISTRIBUTION total wt of polymer Mn total # of molecules M n xi Mi M w wi Mi Mi = mean (middle) molecular weight of size range i xi = number fraction of chains in size range i wi = weight fraction of chains in size range i Degree of Polymerization, DP DP = average number of repeat units per chain H H H H H H H H H H H H H C C (C C ) C C C C C C C C H H H H H H H H H H DP = 6 H H H Mn DP m where m average molecular weight of repeat unit for copolymers this is calculated as follows : m fi mi Chain fraction mol. wt of repeat unit i Types of Polymers • Thermoplastics –Amorphous Crystalline Region –Semi-crystalline • Thermosets Crosslinks Molecular Structures for Polymers secondary bonding Linear Branched Cross-Linked Network Polymers – Molecular Shape Molecular Shape (or Conformation) – chain bending and twisting are possible by rotation of carbon atoms around their chain bonds – note: not necessary to break chain bonds to alter molecular shape Chain End-to-End Distance, r Tacticity Tacticity – stereoregularity or spatial arrangement of R units along chain isotactic – all R groups on same side of chain syndiotactic – R groups alternate sides H H H H H H H H H H H R H H H R C C C C C C C C C C C C C C C C H R H R H R H R H R H H H R H H Tacticity (cont.) atactic – R groups randomly positioned H H H H H R H H C C C C C C C C H R H R H H H R Copolymers two or more monomers polymerized together • random – A and B randomly positioned along chain • alternating – A and B alternate in polymer chain • block – large blocks of A units alternate with large blocks of B units • graft – chains of B units grafted onto A backbone A– B– rando m alternatin g block graft 23 Crystallinity in Polymers • Ordered atomic arrangements involving molecular chains • Crystal structures in terms of unit cells • Example shown – polyethylene unit cell 24 Polymer Crystallinity • Crystalline regions – thin platelets with chain folds at faces – Chain folded structure 10 nm 25 Polymer Crystallinity (cont.) Polymers rarely 100% crystalline • Difficult for all regions of all chains to become aligned crystalline region • Degree of crystallinity expressed as % crystallinity. -- Some physical properties depend on % crystallinity. -- Heat treating causes crystalline regions to grow and % crystallinity to amorphous increase. region Semicrystalline Polymers • Some semicrystalline polymers form spherulite structures • Alternating chain-folded crystallites and amorphous regions • Spherulite structure for relatively rapid growth rates Spherulite surface Photomicrograph – Spherulites in Polyethylene Cross-polarized light used -- a maltese cross appears in each spherulite Adapted from Fig. 14.14, Callister & Rethwisch 8e.