* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download AC Circuits
X-ray photoelectron spectroscopy wikipedia , lookup
Scanning tunneling spectroscopy wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Photoacoustic effect wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Ellipsometry wikipedia , lookup
Particle-size distribution wikipedia , lookup
Chemical imaging wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
Optical coherence tomography wikipedia , lookup
X-ray fluorescence wikipedia , lookup
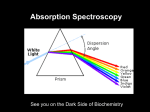
PHYSC 3622 Experiment 2.3 29 June, 2017 Spectrophotometry Purpose In this experiment, you will be introduced to spectrophotometry (optical absorption spectroscopy of solids, liquids and solutions) and will investigate Beer's Law. Background Most materials absorb some light, and the degree to which they absorb light is a function of the wavelength of the light. Because optical absorption in the visible and near-UV portions of the spectrum is generally the result of absorption of light by electrons in atoms, ions or molecules, the absorption characteristics can yield a considerable amount of information regarding their electronic structure. A spectrophotometer is an instrument that measures the amount of optical absorption in a material, as a function of wavelength. There are four main components of a spectrophotomer: 1) A light source. This is usually a tungsten-filament or gas-discharge lamp. Different light sources are used in different regions of the spectrum. 2) A monochromator. The input to the monochromator is the broad-band light from the light source; the output is tunable, highly monochromatic light. 3) A sample chamber. The material under investigation is placed here. 4) A detector. The detector measures the amount of light that passes through the sample. Typically, detectors are either solid-state photodiodes (silicon, germanium, etc.) or photomultiplier tubes. The quantity of interest is the relative amount of light absorbed by the sample. In other words, what fraction of the incoming light is absorbed? The spectrophotometer detector measures the absolute amount of light passing through the material, which is dependent on the emission characteristics of the light source as well as the absorption characteristics of the sample. Therefore, it is necessary to normalize the detector signal by comparing it to the signal obtained when the sample is replaced by a reference material. This reference may simply be air, or, in the case of solutions, the pure solvent. For example, if the detector voltage is 1.50 mV at 532 nm for the reference, and 1.35 mV at 532 nm for the sample, the transmission, T , of the sample is T 1.35 / 1.50 0.90 . (1) Thus, the sample absorbs 10% of the incoming light at 532 nm. (This is sometimes written as T532 to indicate the wavelength.) There are two basic types of spectrophotometers, single-beam and dual-beam. In a single-beam spectrophotometer, reference and sample spectra are obtained sequentially. In a dual-beam instrument, the two spectra are obtained simultaneously. The advantage of the dual instrument is that any time-dependent variations in the intensity of the light emitted by the source are compensated, improving sensitivity and reducing uncertainty. In addition to transmission, another useful way to report the optical absorption is in units of optical absorbance, A . Absorbance is a dimensionless quantity defined as the negative of the base-10 logarithm of transmission: A log 10 T . (2) 1 PHYSC 3622 Experiment 2.3 29 June, 2017 An absorbance of 1 corresponds to a transmission of 0.1, an absorbance of 2 corresponds to a transmission of 0.01, and so on. Absorbance units are useful when working with Beer's Law, which states that the absorbance of a solution is proportional to the concentration, C , of the absorber in that solution: A kC . (3) Most simple molecules obey Beer's Law, particularly at low concentration. Others, such as organic dyes, often exhibit a significant departure from Beer's Law at high concentration. This occurs because at higher concentrations, the molecules begin to interact with each other, and can no longer be treated as independent absorbers. Procedure Initial setup: Turn on the spectrophotometer light source using the inline switch. (The lamp will take about five minutes to warm up and light.) Turn on the power to the monochromator controller labeled SOURCE (the power switch is on the back). Once the light source is operating, verify that the shutter (the flat lever extending towards you) is open (pulled out) and that the iris (the round lever) is set at the maximum aperture (lever vertical). Start the spectrophotometer program by typing RS at the DOS prompt. Once the program begins, note how the different menu items along the left side of the screen can be accessed by using the up- and down-arrow keys. Use the keys to move the pointer to the SMDC Model No entry, and press Enter. Type 3, and then press Enter once again. Verify that the spectrophotometer sample chamber is empty, and that the cover is securely in place. Move the menu pointer to CALIBRATE SYSTEM, and press Enter. This initiates the spectrophotometer's calibration sequence, which will take a couple of minutes to complete. If the SET MODE: menu entry reads TIME BASE, move the menu pointer to it and press Enter to change it to read SCANNING. Holmium oxide filter: Move the menu pointer to SET PARAMETERS and press Enter. Verify that from is set to 300 nm, to is set to 800 nm, SCAN RATE is set to 20 nm s–1 and GAIN is set to 1X. Modify any of the parameters as necessary, and press Esc to return to the main menu. Move the menu pointer to RUN and press Enter. The menu pointer should now point to reference. Press Enter, which will initiate a reference scan. The display will read *** WAVELENGTH RESET IN PROGRESS *** followed by *** READY *** (press any key to begin scan) -ESC to abortPress Enter to begin the scan. The display will read *** SCAN IN PROGRESS *** while the data are accumulated. When the scan is completed, insert the holmium oxide glass filter into the sample holder and replace the sample chamber cover. Move the menu pointer to sample and press Enter to initiate the sample scan. 2 PHYSC 3622 Experiment 2.3 29 June, 2017 When both scans are completed, you can enter identifying information in the sample ID, reference, date and operator fields at the bottom of the menu. Press Esc to return to the main menu. Move the menu pointer to PLOT DATA and press Enter. Most of the settings should be correct, but you will need to change Autorange to OFF and Units to ABS (absorbance). Move the menu pointer to PLOT SCAN DATA and press Enter to view the results of the scan. If you wish, you can modify the horizontal and vertical scales, and then select PLOT SCAN DATA again to update the plot. Press the Print Screen key to print out a copy of the plot. DO NOT use the PRINT menu item, as this will lock up the program. Holmium oxide ( H 2 O 3 ) exhibits seven major absorption peaks in the visible and nearUV portions of the electromagnetic spectrum, at 360.9, 418.7, 445.8, 453.2, 460.0, 536.2 and 637.5 nm. The origin of these peaks is optical absorption by electrons occupying the partially-filled 4f levels of the Ho 3+ ion. Identify and label each of these peaks on your plot. Take more detailed scans of the 360.9, 445.8 and 536.2 nm peaks. For example, you might want to scan over 350–370 nm, at a scan rate of 1 nm s–1 for the 360.9 nm peak. Measure and record the full-width half maximum (FWHM) for each of these three peaks. The FWHM is defined as the width of the peak measured at one-half of the peak height. Beer's Law: In this part of the experiment, you need to measure the absorption spectrum of the Rose Bengal solution at different concentrations (Rose Bengal is an organic dye). Prepare a master aqueous solution of molar concentration ~1.25x10-4 M (moles/l) in a large storage vial. Use the plastic pipettes to transfer the master solution from the storage vial to 9 smaller vials and add distil water to make 9 dilute solutions in the concentration range between ~1.25x10-4 M and ~5x10-7 M. You may need 1 sample in the 10-4 M range, 3 samples in the 10-5 M range, 4 samples in the 10-6 M range, and 1 sample in the 10-7 M range. Use the plastic cuvettes to hold the sample solutions, and use the plastic pipettes to transfer the solutions from the storage vials to the cuvettes. To avoid contamination, use a new pipette and new cuvette for each sample. After each measurement, discard the solution in the cuvette (do not return it to the storage vial). Use a cuvette filled with water as the reference for each solution. After obtaining the spectra for each of the solutions, plot the absorbances of the main peak and the shoulder peak as a function of concentration. Using Sigma Plot to determine the best-fit straight line through your data points. Questions Assume that the narrowest absorption peak in the Ho 3+ spectrum is actually infinitesimally wide, so that the FWHM that you measure represents the resolution of the instrument. How closely can two peaks be spaced and still be resolved? Does Rose Bengal obey Beer's Law? 3