* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CHAPTER 3 ESSENTIALS OF METABOLISM
Size-exclusion chromatography wikipedia , lookup
Signal transduction wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemical cascade wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Biosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Metalloprotein wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biochemistry wikipedia , lookup
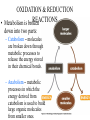
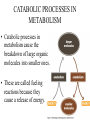
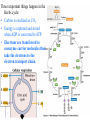
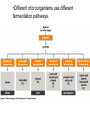
CHAPTER 3 ESSENTIALS OF METABOLISM Photo courtesy of Dr. Brian Oates WHY IS THIS IMPORTANT? • It is important to have a basic understanding of metabolism because it governs the survival and growth of microorganisms. • The growth of microorganisms can have a direct effect on infectious disease. • Good metabolic function makes pathogens more successful at causing disease. OVERVIEW BASIC CONCEPTS OF METABOLISM • Metabolism is: – A series of chemical processes that go on in living organisms. – Used to obtain energy. – Linked to growth. BASIC CONCEPTS OF METABOLISM • Carbon and energy are required for growth. • There are two processes by which carbon can be obtained: – Autotrophy – carbon is obtained from inorganic substances (e.g. plants using CO2 to make sugar) – Heterotrophy – carbon is obtained from other organic molecules • Nearly all infectious organisms are chemoheterotrophs. • Chemoheterotrophs obtain energy by breaking down other organic molecules and compounds. OXIDATION & REDUCTION REACTIONS • Metabolism is broken down into two parts: – Catabolism – molecules are broken down through metabolic processes to release the energy stored in their chemical bonds. – Anabolism – metabolic processes in which the energy derived from catabolism is used to build large organic molecules from smaller ones. OXIDATION & REDUCTION REACTIONS • Both anabolism and catabolism involve electron transfer and oxidation and reduction reactions – redox reaction. • An oxidation reaction is a chemical reaction in which an atom, ion or molecule loses one or more electrons. • A reduction reaction is a chemical reaction in which an atom, ion or molecule gains one or more electrons. OXIDATION & REDUCTION REACTIONS • Oxidation and reduction reactions always occur together. – The combination of an oxidation reaction and a reduction reaction are jointly referred to as redox reactions. • When a substance is oxidized, it loses electrons. • When a substance is reduced, it gains electrons. RESPIRATION • In metabolism, respiration occurs at the cellular level and is not the same as breathing (respiration at the macroscopic level). • Cellular respiration describes catabolic processes and is divided into: – Aerobic respiration – metabolism that uses oxygen – Anaerobic respiration– metabolism that does not use oxygen – Facultatively anaerobic respiration – metabolism that can use oxygen but can also occur without it METABOLIC PATHWAYS • Metabolic reactions occur in series of chemical reactions called pathways. – The following is an example of a pathway. A is the initial substrate and E is the final product of the pathway, with B, C, and D being intermediates. A B C D E • Each step in the pathway is mediated or facilitated by a specific enzyme. ENZYMES • Enzymes are proteins that act as catalysts for metabolic reactions, making the reaction go faster. • Enzymes work by lowering the energy of activation. • Each enzyme is specific for a reaction. •Enzymes are found in all living organisms and most cells contain hundreds of types which are constantly being manufactured and replaced. PROPERTIES OF ENZYMES • Enzymes have specific three dimensional shapes: if the shape changes, activity is inhibited. • The shape of the molecule provides a distinctive site called the active site. It is here that: – The substrate fits into the enzyme’s active site. – The enzyme and substrate interact to form the enzymesubstrate complex. • The active site has to have the proper shape for the enzyme to work. PROPERTIES OF ENZYMES • Enzymes are generally highly specific. • A given enzyme catalyzes only one type of reaction. • Most enzymes react with only one particular substrate. • The shape and electrical charges found at the active site allow for the reaction to work and are responsible for the enzyme’s specificity. PROPERTIES OF ENZYMES • Some enzymes work on more than one substrate but in these cases the enzymes always work in a particular type of reaction. – A proteolytic enzyme always degrades proteins because it reacts only with peptide bonds. COENZYMES AND CO-FACTORS • Many enzymes can catalyze a reaction only if other substances are present at the active site. – These enzymes are referred to as apoenzymes. • Co-factors are helper substances that are inorganic ions such as magnesium, zinc, or manganese. • Coenzymes are helper substances that are non-protein organic molecules. • Co-factors or coenzymes bind to the active site and change the shape of the active site so the substrate now fits. COENZYMES AND CO-FACTORS • Coenzymes and co-factors can also be used as carrier molecules. – When a carrier molecule receives either electrons or hydrogen atoms, it becomes reduced. – When a carrier molecule releases electrons or hydrogen atoms, it becomes oxidized. COENZYMES AND CO-FACTORS • Two coenzyme carrier molecules frequently encountered in biological reactions are: – NAD+ = nicotinamide adenine dinucleotide – FAD = flavin adenine dinucleotide. ENZYME INHIBITION • Enzyme inhibition takes place in three ways: – Competitive inhibition – Allosteric inhibition – Feedback inhibition COMPETITIVE INHIBITION • The inhibitor molecule is similar in structure to the substrate and competes with the substrate to bind to the active site. • When the inhibitor has bound to the active site, the substrate cannot bind. •The binding of the competitor is reversible and dependent upon the relative numbers of inhibitor molecules and substrate molecules present. ALLOSTERIC INHIBITION • Inhibitor molecules bind to a part of the enzyme away from the active site: the allosteric site. • This binding changes the shape of the active site in such a way that it can no longer fit properly with the substrate. • The binding of some allosteric inhibitors is reversible. FEEDBACK INHIBITION • The final product in a pathway accumulates and begins to bind to and inactivate the enzyme that catalyzes the first reaction of the pathway. • It is reversible and, when the level of end product decreases, the inhibition stops and the pathway begins to function again. FACTORS THAT AFFECT ENZYME REACTIONS • Three major factors affect enzyme activity: – Temperature – Can break hydrogen bonds and change shape – pH – Can break hydrogen bonds and change shape – Concentration of substrate, product & enzyme – Lower numbers of substrate, product, and enzyme molecules means a lower level of activity. CATABOLIC PROCESSES IN METABOLISM • Catabolic processes in metabolism cause the breakdown of large organic molecules into smaller ones. • These are called fueling reactions because they cause a release of energy. CATABOLIC PROCESSES IN METABOLISM • There are three important pathways by which most organisms release energy from nutrient molecules: – Glycolysis – Krebs cycle – Electron transport chain GLYCOLYSIS • The catabolic pathway used by most organisms. • The best example of this pathway is glucose breakdown. • The process itself is a series of chemical reactions. Glucose • Glycolysis occurs in the cytoplasm and does not require oxygen. • Four ATP molecules are produced in glycolysis – The first steps of the pathway consume two ATP molecules. – The net gain is two ATP molecules. 4 – 2 = 2 ATP produced Per glucose molecule (Net) 1 molecule of glucose Produces: – After a series of steps, the 6-carbon glucose molecule 2 ATP (net) broken into two 3-carbon pyruvate molecules -> 2 reducedisNAD+ 2 molecules of Pyruvate Krebs cycle. – NAD+ carries electrons to the electron transport chain. • Glycolysis can lead to further energy producing pathways. – Krebs cycle and cellular respiration (aerobic) – Fermentation (anaerobic) THE KREBS CYCLE • The Krebs cycle, A.K.A. the citric acid cycle or TriCarboxylic Acid cycle (TCA). • It is an aerobic catabolic pathway seen in aerobic cellular respiration. • Pyruvate is further metabolized in this process. • Pyruvate is oxidized to reduce NAD+ and modified with coenzyme A to produce Acetyl-CoA complex. THE KREBS CYCLE • The Krebs cycle is a series of reactions in which chemical changes occur. – Within these reactions, hydrogen atoms are removed and their electrons are transferred to coenzyme carrier molecules. – The hydrogen atoms are carried by NAD+ and FAD to the electron transport system. Three important things happen in the Krebs cycle: • Carbon is oxidized as CO2. • Energy is captured and stored when ADP is converted to ATP. • Electrons are transferred to coenzyme carrier molecules that take the electrons to the electron transport chain. ELECTRON TRANSPORT CHAIN • The electron transport chain is a sequence of molecules. – In eukaryotes, they are found in the inner mitochondrial membrane. – In prokaryotes, they are organized in the plasma membrane. ELECTRON TRANSPORT CHAIN • Electrons are transferred to a final electron acceptor. – In aerobic respiration, the final acceptor is oxygen. – In anaerobic respiration, the final acceptor is an inorganic oxygen-containing molecule. CHEMIOSMOSIS • As electrons move from one molecule to another in the chain, energy is released via a process called chemiosmosis. • As electrons are transferred along the electron transport chain, protons are pumped out of the cell. • This causes the proton concentration outside the cell to be higher than inside the cell, causing a concentration gradient to form. CHEMIOSMOSIS • Specialized membrane proteins allow protons to re-enter the cell. – Energy is released as protons re-enter the cell. – This energy is used to bind phosphate to ADP, making the highenergy molecule ATP. – The difference in proton concentration in this process is called the proton motive force. CHEMIOSMOSIS • Cells using anaerobic respiration generate two molecules of ATP from one glucose molecule. • Cell using aerobic respiration generate thirty eight total molecules of ATP from one glucose molecule. Aerobic respiration: 38 ATP / glucose molecule Anaerobic respiration: 2 ATP / glucose molecule FERMENTATION • Fermentation is the enzymatic breakdown of carbohydrates in which the final electron acceptor is an organic molecule. •Different microorganisms use different fermentation pathways. ANABOLISM • Anabolic reactions are classified as biosynthetic reactions because they are used to synthesize all the biological molecules needed by the cells of living organisms. • Biosynthetic reactions form the network of pathways that produce the components required by the cell for growth and survival. • These reactions are fueled by the energy stored in high-energy bonds in ATP. ANABOLISM