* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download SBI3U
Survey
Document related concepts
Signal transduction wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Point mutation wikipedia , lookup
Metalloprotein wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Proteolysis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Protein structure prediction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Transcript
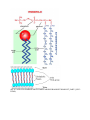
SBI3U ORGANIC MOLECULES are generated in living things are carbon based molecules Note: CARBON can form 4 covalent bonds making it the “backbone atom” of organic compounds refers to molecules contain BOTH carbon and hydrogen can also contain oxygen, nitrogen, sulfur, and/or phosphorus Recall: Inorganic Molecules examples: O2, H2O, CO2 4 Major Groups of Organic Macromolecules Carbohydrates Lipids Proteins Nucleic acids Macromolecules are large molecules (polymers) composed of smaller subunits (monomers) bonded together CARBOHYDRATES Basic Formula: CnH2nOn e.g. Glucose C6H12O6 carbo hydrate where n can be any whole number THEIR FUNCTION Short and long-term energy storage (e.g. glucose, starch) Physical strength to cell structure (e.g. cellulose – makes cell walls) THEIR STRUCTURE 3 major classes of carbohydrates (CHOs): Monosaccharide (single sugar) simple sugar (monomer; smallest unit of CHOs) one CHO molecule that has 3-7 carbon atoms e.g. glucose, fructose, galactose, ribose glucose (diagram below) Disaccharide (double sugar) composed of two simple sugars linked together e.g. maltose, lactose, sucrose (http://staff.jccc.net/PDECELL/biochemistry/sucrosesyn.gif) Polysaccharide (many sugar) composed of many simple sugar molecules linked together e.g. Starch – energy storage in plants Glycogen – energy storage in animals (liver and muscle) Cellulose – structural molecule in cell walls (http://kentsimmons.uwinnipeg.ca/cm1504/carbohydrates.htm) LIPIDS are very diverse common feature do not dissolve in water (they are nonpolar) hydrophobic water hating THEIR FUNCTION Long-term energy and nutrient storage Insulation Cushioning of internal organs Hormones (chemical signals that send messages around the body) TYPES and STRUCTURES 1. FATS – solid at room temp. 2. OILS – liquid at room temp. both made up of GLYCEROL and 3 FATTY ACIDS Fatty acids may be: Saturated has no double bonds (present in fats) Unsaturated has double bonds (present in oils) General Structure: (known as a triglyceride) 3. PHOSPHOLIPIDS – important in the structure of cell membranes made up of a glycerol, PHOSPHATE, and 2 fatty acids “head” is polar (hydrophilic) “tail” is nonpolar (hydrophobic) General Structure: (HTTP://WWW.ELSOMRESEARCH.COM/LEARNING/BOOKLET/BOOKLET_PART4_SEC1. HTML) PROTEINS most diverse and complex THEIR FUNCTION Provide structure (e.g. hair, bones, muscle) Facilitate chemical reactions as enzymes (e.g. amylase in saliva) Transport substances (e.g. across cell membrane, hemoglobin in blood) Act as chemical messenger (e.g. insulin regulates glucose concentration) THEIR STRUCTURE Made up of monomers called amino acids (20 different a.a.) General Structure: (http://www.hcc.mnscu.edu/programs/dept/chem/V.27/amino_acid_structure_2.jpg) *See page 14 in your textbook. amino acids are linked by peptide bonds to form a polypeptide polypeptide folds into a 3-D structure to form a very specific shape *See page 16 in your textbook. Note: SHAPE of a protein determines its FUNCTION! NUCLEIC ACIDS THEIR FUNCTION Determine what characteristics living things have (e.g. DNA) Direct protein synthesis within the cell Energy carrier within the cell THEIR STRUCTURE Monomer is called a nucleotide (nt) made up of a phosphate, sugar, and nitrogenous base (http://www.chemsoc.org/ExemplarChem/entries/2003/imperial_Burgoine/nucleotide.jpg) http://www.msu.edu/course/isb/202/ebertmay/notes/snotes/02_13_07 _genes_evo1.html http://www.emc.maricopa.edu/faculty/farabee/BIOBK/BioBookDNAMOLGEN.html Polymer can be: a. RNA – ribonucleic acid single strand of nucleotides each nt contains ribose sugar b. DNA – deoxyribonucleic acid double stranded nucleotides each nt contains deoxyribonucleic acid Note: There are 4 different nucleotides (due to different bases) DNA adenine, guanine, cytosine, thymine RNA adenine, guanine, cytosine, uracil DNA structure: (HTTP://FIG.COX.MIAMI.EDU/~CMALLERY/150/GENE/16X5BC.JPG)