* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download classification of matter - St. Thomas the Apostle School
Chemical Corps wikipedia , lookup
Chemical industry wikipedia , lookup
Thermal spraying wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Chemical plant wikipedia , lookup
Condensed matter physics wikipedia , lookup
Chemical potential wikipedia , lookup
Stöber process wikipedia , lookup
Photopolymer wikipedia , lookup
Ceramic engineering wikipedia , lookup
Freeze-casting wikipedia , lookup
Nanochemistry wikipedia , lookup
Colloidal crystal wikipedia , lookup
Nanoparticle wikipedia , lookup
History of chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Elementary particle wikipedia , lookup
State of matter wikipedia , lookup
Mineral processing wikipedia , lookup
Sol–gel process wikipedia , lookup
Safety data sheet wikipedia , lookup
Particle-size distribution wikipedia , lookup
Atomic theory wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
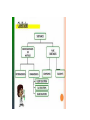
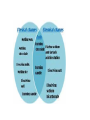
CLASSIFICATION OF MATTER Chapter 15 Composition of matter • Substance- either an element or a compound • When all the atoms in a substance are alike, the substance is an element. • A compound is a substance with two or more elements combined in a fixed proportion. Two or more substances that can be easily separated by physical means form a mixture. 1. Heterogeneous mixture- mixture of different and easily distinguishable materials. 2. Homogeneous mixture- contains two or more gaseous, liquid, or solid substances blended evenly; also called a solution. 3. Colloid- heterogeneous mixture with larger particles that never settle; colloids scatter light in the Tyndall Effect. 4. A heterogeneous mixture containing a liquid in which visible particles settle is called a suspension. Mixtures HETEROGENEOUS-proportions of substance may vary -different materials can be identified - Particles settle in a suspension HOMOGENEOUS- Particles are too small to be seen with a microscope. - Particles never settle - They remain constantly mixed COLLOID-particles are larger than in sollutions -Contains substances in varying proportions -particles wil not settle Particles scatter light The Tyndall Effect The Tyndall effect, also known as Tyndall scattering, is light scattering by particles in a colloid or else particles in a very fine suspension. It is named after the 19th-century physicist John Tyndall. On a sheet of paper give three physical properties of yourself. Properties of matter •Physical Property- characteristics of a material which can be observed without changing the identity of the substance in the material. ; examples include color, shape, size, melting point, and boiling point Physical Properties: •Appearance- physical description of a substance •Behavior- how a substance acts; for example magnetism, viscosity, ductility •Physical properties such as size and magnetism can be used to separate mixtures. Physical Change- change in a substance’s size, shape, or state of matter • Substance does not change identity when it undergoes a physical change • Distillation is a process for separating a mixture by evaporating a liquid and condensing its vapor. CHEMICAL PROPERTY- characteristics of a substance indicating that it can change chemically • For example- flammability or light sensitivity of a substance When one substance changes to another substance, a chemical change has occurred. • Some chemical changes are indicated by temperature change, smell, or bubble formation. • Other chemical changes occur very slowly such as the formation of rust. • Chemical changes can be used to separate substances such as metals from their ores. • THESE PROPERTIES of a substance NEVER CHANGE: density, specific heat, boiling point, melting point WEATHERING of Earth’s surface involves both physical and chemical change. • Physical- big rocks split into smaller ones; streams carry rock particles from one location to another. • Chemical- Chemical change can occur in rocks when calcium carbonate in limestone changes to calcium hydrogen carbonate due to acid rain. Law of Conservation of mass- mass of all substances present before a chemical change equals the mass of all substances after the change.