* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Electron configuration of atoms
Survey
Document related concepts
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
James Franck wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Wave–particle duality wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Tight binding wikipedia , lookup
Electron configuration wikipedia , lookup
Hydrogen atom wikipedia , lookup
Transcript
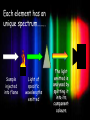
Electron configuration of atoms Shells, sub-shells and orbitals Syllabus point: 2.2.1 a,b,c,d HSW 1 & 7 A level SYLLABUS Bohr Model of the Atom • Recall that Hydrogen’s atomic emission spectrum is discontinuous, or made up of only certain frequencies of light. • Why? • Niels Bohr proposed a quantum model for Hydrogen that seemed to answer this question. Bohr’s Model Cont. •Bohr built on Planck’s and Einstein's concepts of quantized energy (only certain values are allowed). •Bohr proposed that the Hydrogen atom only has certain allowable energy states. •The lowest allowable state was termed: ground state. Bohr’s Model Cont. •When an atom gains energy, it is said to be in an excited state. •Even though Hydrogen contains only one electron, it can have many excited states. •Bohr continued to expand his model by stating that electrons found closer to the nucleus had less energy than electrons found at greater distances from the nucleus. Bohr’s Model Cont. Bohr assigned a quantum number, n, to each shell/energy level: • n1 • n2 • n3 • Etc. http://www.biologydaily.com/biology/upload/thumb/d/de/307px-Bohratommodel.png Bohr’s Explanation of Hydrogen’s Spectrum • When a Hydrogen atom is in the ground state, n=1, it does not radiate any energy. • When energy is added from an external source, the electron moves to a higher energy orbit. • The atom is now in an excited state. • The electron then drops to a lower state and emits a photon corresponding to the difference between the energy levels of the two orbits. How can we apply this theory to explain atomic spectra? We know that white light can be split into a spectrum by passing it through a prism. After the sunlight had been broken down into its components by one prism, if a narrow ray of the light from the first prism was passed through another prism there would be no further breakdown. Joseph von Fraunhofer is best known for his discovery of the dark absorption lines known as Fraunhofer lines in the Sun's spectrum, and for designing achromatic telescope objectives. In 1814, he developed a spectroscope to study the spectrum of the light given off by the sun. He was amazed to discover that in the midst of the rainbow of colors was a series of black lines. Joseph von Fraunhofer (March 6, 1787 – June 7, 1826) These dark lines were later determined to be the result of the absorption of selected frequencies of the electromagnetic radiation by an atom or molecule. Bunsen and Kirchhoff further developed the spectroscope by incorporating the Bunsen burner as a source to heat the elements. In 1861, experiments by Kirchhoff and Bunsen demonstrated that each element, when heated to incandescence, gave off a characteristic color of light. When the light was separated into its constituent wavelengths by a prism, each element displayed a unique pattern or emission spectrum. Emission Spectra Complement Absorption Spectra The emission spectrum seemed to be the complement to the mysterious dark lines (Fraunhofer lines) in the sun's spectrum. This meant that it was now possible to identify the chemical composition of distant objects like the sun and other stars. They concluded that the Fraunhofer lines in the solar spectrum were due to the absorption of light by the atoms of various elements in the sun's atmosphere. Each element has an unique spectrum…….. Sample injected into flame Light of specific wavelengths emitted The light emitted is analysed by splitting it into its component colours Atomic Emission Spectra •When electricity is passed through a tube of gas, the atoms in the tube absorb energy and become excited. •The atoms release the energy absorbed in the form of light. •Each atom has specific frequencies it will release in the light form. Examples of emission spectra Hydrogen Oxygen Sodium Potassium Flame Tests Flame Test: A test used in the identification of certain elements. It is based on the observation that light emitted by any element gives a unique spectrum when passed through a spectroscope. Flame spectrum for lithium. (Notice the faint bands of color in the spectra.) Analysis and Detection Using Atomic Emission Spectroscopy Development Aided by the rapid progression in technologies such as electronics and computing. Modern machines will do the analysis and interpret the results. Atomic Emission Spectroscopy Advantages: Very quick Automated process Only requires a small sample, the brightness of the spectrum will determine how much sample there is (concentration) Atomic Emission Spectroscopy Disadvantages: Destructive – the sample being tested is burned! Only identifies the presence of elements – does not identify compounds. Usually need spectra of known compounds to compare to unknown results Analytical machines can be expensive and only suitable for particular tasks Applications quality control in chemical manufacture e.g. potassium compounds often have sodium contamination – atomic emission spectroscopy could determine if any sodium was present and how much. Working out the composition of distant stars Applications Detection of alcohol, drugs or metal ions in the blood Detect impurities in products that need to be pure, such as drug molecules and alloys Detect pollutants in water, soil and air However……….. •It was eventually found that Bohr was incorrect. •Electrons do not travel in circular orbits around the nucleus. •Poor Bohr!