* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 19 Oxidative Phosphorylation-Electron Transport A
Survey
Document related concepts
Basal metabolic rate wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Mitochondrion wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
Transcript
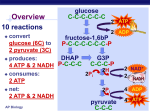
19 Oxidative Phosphorylation-Electron Transport A/P Biology Pages 172-182 Name: ________________________ Date: _______________ Period: __________ If we take a look at the over-all picture of Glycolysis- we can see that this process that requires no oxygen, releases a few high energy products (ATP/NADH/pyruvate): When we look at the over-all picture of the Krebs cycle we see more high energy products being produced: The object is to capture as much energy as possible (as ATP) from these high energy products. Electron Transport is a construct of a series of proteins embedded in the mitochondrial membrane. The mitochondrion looks like this in a simplistic picture: Page 2 (Cont. #19) The key is looking at this rather complex organelle up close-at the level of the “matrix” and the “inter-membrane space”. Embedded in the “inner membrane” are a number of protein complexes that are used to step down the energy provided by either NADH or FADH2. What is meant by this “stepdown” is the relative energy from the proton/electron donor (NADH/FADH2) and the final acceptor of those protons and electrons, oxygen. The diagram to the left is merely to show the step-down of the amount of energy the electrons have allowing the protons to be expelled from the matrix and shunted to the “inter membrane” space. The “*” denotes when the electron loses enough energy to couple the movement of a proton from the matrix to the inter-membrane space. The final acceptor of the “formally” excited electron is oxygen. A constant supply of oxygen (by breathing) is necessary for each and every cell to allow the diffusion of oxygen into the cell and then into the mitochondria for THIS specific purpose! If we deprive the cell of oxygen- the electron transport backs up and halts the production of ATP for each and EVERY mitochondria. This shuts them down. You can guess that the cell will run out of ATP molecules and shut down as well. Page 3 (Cont. #19 A/P Biology) Another view of the relationship of the Krebs Cycle and the electron transport chain embedded in the “inner-membrane” (see diagram of mitochondria, page 2 of this handout) of the mitochondria looks like this: Let us take a closer look at an artists rendering of just a small part of this electron transport: 1.) NADH binds to the first enzyme complex called NADH-Q reductase (also called “complex 1”) One proton is pumped out. (see H+) 2.) The electrons are then transferred to Coenzyme Q (also called Ubiquinone). This is often referred to as “Complex 2”. One proton is pumped out. Page 4 (Cont. #19 A/P Biology) 3.) Electrons are transferred from Coenzyme Q to Cytochrome “bc” complex also known as “Complex 3”. 4.) Electrons are then transferred to Cytochrome “C” which is a peripheral membrane protein (i.e. it sits on top of the inner membrane on the inter-membrane space side. This protein is somewhat famous because it is relative easy to study AND because the primary structure of the protein is “Highly Conserved” throughout many different organisms. That means that the amino acid sequence in a rat’s mitochondria is strikingly similar to that found in a human’s mitochondria and mitochondria found in single-celled eukaryotes. 5.) Cytochrome C transfers its electrons to the Cytochrome oxidase complex (also called “Complex 4”). The electrons are passed to the matrix where OXYGEN accepts the electrons and one proton is pumped out. The pumping of the protons across the membrane establishes a “Proton Gradient” across the inner membrane. The energy stored in the proton gradient is used to drive the ATP synthesis reaction as the protons flow back to the matrix through Complex 5 (ATP synthetases- there are more than one- in fact there are hundreds per mitochondria). You can see animation of the Glycolytic cycle, Krebs Cycle, and electron transport pumping synthesis in action by going to: You tube and searching for Glycolysis or Krebs or Electron Transport and watching/listening to the videos presented! To summarize the complete oxidative respiration: Without the presence of oxygen, there can be no receptor for the electrons within the mitochondrial matrix and the electron transport chain will rapidly shut down. Without a constant supply of ATP there are NO cells that can sustain function (that is remain organized in the process of using ATP)! FADH2 does not create as many ATP molecules Page 5 (Cont. #19 A/P Biology) compared to NADH because it contributes its hydrogen and electrons at a slightly lower energy level (to Coenzyme Q (CoQ … see picture pg. 2) than NADH. The proton will then be donated to Cyctochrome b/c will then follow the same route of electron transport and proton pumping as NADH. That means there is one less proton being pumped out of the matrix and into Inter membrane space when FADH2 donates the proton. That equates to one less ATP molecule being made. So where does the oxygen come from for the electrons and the protons to combine with to make water? All organisms (heterotrophs and autotrophs alike) MUST take in oxygen so that it can be the receptor for the electron in the electron transport IF the organism HAS mitochondrion! Bacteria and yeast still require a little oxygen to allow NADH to be oxidized to replenish the necessary high energy compound (NAD+) to allow glucose to be broken down to pyruvate (see overall picture of Glycolysis – handout #17note NAD+ is necessary to transform glyceraldehydes-3-phosphate to 1,3-bisphosphoglycerate)! Carefully scan the diagram and follow the production of NADH and then the production of NAD+ after pyruvate is made! The take-home message here is not to have you memorize these steps, rather understand the basic flow of high energy compounds as they are produced and degraded, what “waste products” are formed and how much energy (ATP) is produced over-all. The act of cellular respiration answers two key questions about life: 1.) Why is it necessary we breathe in OXYGEN? …to accept the electron in the electron transport chain, and that electron transport chain powers the production of ATP that runs nearly all reactions that require energy in the cell. 2.) Why is carbon dioxide a waste product and why do we make so much of it? …you lose all of the carbons of glucose during the breakdown of pyruvate to acetyl-CoA and acetyl-CoA through the Krebs cycle! So you see there is a certain conservation of atoms here. Oxygen is taken in and transformed into water. Sugar is taken in and is released as carbon dioxide and ATP (of course a lot of heat is lost in this process…but it is the cost of living). A recent article in Popular Science Magazine (Feb. 2009) entitled “Freezing the Heart to Save Lives” brings the dynamics of oxygen as a required molecule not only for its position to accept the final electron in the electron transport chain, but also its toxicity if the cell is without oxygen for a while. If we examine what happens to a heart attack patient or a victim of drowning, we see that by some means oxygen does not flow to individual cells. All the electron transport chains still run at full tilt transferring electrons to Cytochrome A in every single mitochondrion in every cell of the body! Rescuers or doctors try to revive the drowning victim or the heart attack patient. What happens? As soon as oxygen becomes available, every single Cytochrome A molecule tries to release all of their electrons to a relatively few oxygen molecules creating “free radicals” (oxygen molecules with extra electrons). As stated in the article, “When oxygen flow returns, the mitochondria start producing free radicals; other cellular ion levels also go awry. The injured cells start dying, and in response, the immune system releases chemicals (sic: interleukins- cell signals to attract more immune response cells) that worsen the effect. The problem is most pronounced in the heart and the brain, which use Page 6 (Cont. #19 AP Biology) more oxygen that other organs.” In other words while trying to save someone who has stopped breathing- you naturally try to restore oxygen to all the cells. If too much time goes by, the back up in the electron transport chain becomes over-whelming. How are doctors trying to solve this problem? They cool the body because, “…the mitochondria and the immune system are not as active at low temperatures.” There is still a lot of research that needs to be worked out for a standard procedures and reasonable outcomes for heart attack victims or victims of drowning. The gist of the argument lies in the absolute requirement for oxygen to accept electrons – and realizing what might happen to the cell if it is starved of that oxygen for a period of time. Cooling the body (rapidly) MAY (and seems to) help because it might prevent the build up of electrons in the electron transport chain. However, the risks of hypothermia to the body as a whole must be considered as well. Two more key questions are: 1.) What happens to the water that is produced? and 2.) What happens to the carbon dioxide that is produced as waste? We will answer these questions as part of the next handouts discussing photosynthesis! After reading the required text and this handout…answer the following questions here and on your scan-tron. _____ 1.) Look at the picture on page 1 of this handout. How many NET ATPs are produced by glycolysis? a.) 1 b.) 2 c.) 3 d.) 4 e.) 6 _____ 2.) How many oxygen molecules are required to allow the cell to undergo glycolysis? a.) Zero b.) Two c.) Four d.) Six e.) Eight or more _____ 3.) Each molecule of glucose that undergoes glycolysis produces how many pyruvate molecules? a.) 1 b.) 2 c.) 3 d.) 4 e.) 6 _____ 4.) Other than pyruvate and ATP, what other “high energy product” is produced by glycolysis? a.) water b.) glucose c.) sucrose d.) deoxyribose e.) NADH _____ 5.) When pyruvate (a three carbon molecule) enters the mitochondria, what is really happening (for our purposes) is that two pyruvate are entering the mitochondriai.e. to account for all SIX carbon of glucose. One carbon dioxide molecule is removed from pyruvate as it enters through the mitochondrial membrane (lost as CO2).The Krebs cycle removes two more carbons as CO2. How many turns of the Krebs cycle are needed to release all 6 carbons from glucose? a.) 1 b.) 2 c.) 3 d.) 4 e.) 6 Page 7 (Cont. #19 AP Biology) _____ 6.) Speculated why there are so many “cristae” in the mitochondrial membrane? a.) there has to be “canals” that move products from place to place, just like endoplasmic reticulum b.) the electron transport chain complex is embedded in the inner membrane and the more surface area of that membrane the more electron transport can be undertaken c.) the “cristae” give the mitochondria its unique “look” so we can tell the difference between it and other organelles d.) the mitochondria has no “cristae”, rather several organelles are found inside the mitochondria e.) none of the above are correct _____ 7.) To create ATP, the extra protons (and electrons) MUST be accepted by WHAT molecule to produce water? a.) water b.) sulfur c.) phosphorous d.) oxygen e.) glucose _____ 8.) What “DRIVES” the production of ATP as a result of the electron transport? a.) the proton gradient from the inter-membrane to the matrix- using ATP synthase b.) the water production c.) the carbon dioxide production d.) the NADH production e.) the glucose production _____ 9.) Theoretically, EACH NADH produces three ATP and EACH FADH2 produces 2 ATP molecules. Glucose is broken down using the glyocolytic cycle and the Krebs cycle. NADH/FADH2 is oxidized via the electron transport chain. Go back through Both diagrams and determine how many NET ATP molecules should be produced during the breakdown of one molecule of glucose (remember, two molecules of ATP are used up at the beginning of glycolysis!). a.) 30 ATP b.) 32 ATP c.) 34 ATP d.) 36 ATP e.) 40 ATP _____ 10.) Look at the over-all reaction on page 4 of this handout. Where is water produced as a product? a.) When glucose is broken down b.) When pyruvate is broken down to acetyl-CoA c.) At the end of the electron transport chain d.) All of the above are correct _____ 11.) According to the first picture on page three, where does the Krebs Cycle take place? a.) in the cytoplasm b.) in the inter-membrane space of the mitochondria c.) in the inter-membrane of the mitochondria d.) in the matrix of the mitochondria e.) outside of the cell altogether Page 8 (Cont. #19 AP Biology) _____ 12.) Why is it you can’t deprive yourself of oxygen for more than a few minutes? a.) oxygen is needed to initially breakdown glucose in the cytoplasm b.) oxygen is needed to breakdown pyruvate to acetyl-CoA c.) oxygen is needed to allow acetyl-CoA to enter the Krebs cycle d.) oxygen is needed during the Krebs cycle e.) oxygen is needed at the end of the electron transport chain _____ 13.) Cytochrome “C” is a “surface” protein found on the outer portion of the innermembrane of the mitochondria. The actual amino acid sequence is highly conserved. Which statement(s) is(are) reasonable to make? a.) the three dimensional structure of the protein is conserved b.) the structure/shape of the molecule MUST remain conserved to accept the electron c.) the structure/shape of the molecule MUST remain conserved to pass along the electron d.) all of the above are correct _____ 14.) Look at the first diagram on page 3 of this handout. Protons (H+) are pumped from the matrix to the inter-membrane space of the mitochondria. What provides the driving force that pushes the protons into the inter-membrane space? a.) the movement of water into the matrix b.) the movement of water out of the matrix c.) the movement of electrons from a high energy compound to a lower energy compound d.) the movement of NAD from a high energy compound to a lower energy compound e.) none of the above explains how the protons are pumped out _____ 15.) What force causes the production of ATP from ADP plus inorganic phosphate in the inter membrane of the mitochondria? a.) protons moving from the inter-membrane space to the matrix through ATP synthetase b.) protons moving from the inter-membrane space to the outside of the mitochondria c.) protons moving from the matrix to the inter-membrane space d.) light hitting the mitochondria e.) all of the above contribute to the production of ATP Answer the following questions on this sheet only! _____ 16.) What is substrate level phosphorylation? a.) the generation of ATP by the direct transfer of a phosphate group to ATP from another phosphorylated molecule using and enzyme b.) the generation of ATP from inorganic phosphate and ADP using ATP synthetase c.) the generation of glucose by reversing glycolysis (gluconeogenesis) d.) it is the generation of NADH by oxidizing ATP e.) none of the above are correct Page 9 (Cont. Handout #19 AP Biology) _____ 17.) Which macromolecule, a single molecule of glucose or a single molecule of a long chain fatty acid produces more ATP molecules (pg. 179)…? a.) glucose b.) fatty acid c.) they both produce the same amount of ATP molecules _____ 18.) Speculate- what would you expect to happen to a muscle cell that is forced to work harder with each and every work-out? a.) more glycolytic enzyme complexes will be made b.) more lactic acid will be produced c.) more mitochondria will be produced d.) all of the above are likely _____ 19.) Speculate- what would you expect to happen to a muscle cell that over time has fewer demands on it? a.) less glycolytic enzyme complexes would be produced b.) less lactic acid would be produced c.) less mitochondria would be produced d.) all of the above are likely ______ 20.) Would you ever expect there to be a “regulation” (that is adding more or less electron transport chains) to the mitochondrial membranes based on energy demands? (Pick the BEST answer!) a.) yes, because if there is increased energy demands then the cell needs to produce more electron transport chains to meet demand b.) yes, there would be an increase in the number of electron transport complexes limited by space available in the membrane c.) no, extra electron transport chains would require too much energy to produce- that is you would not get enough return to make it worthwhile for the mitochondria d.) no, the extra electron transport chains might destabilize the membrane Answer the following questions on this sheet only! 21.) According to the text, the complete oxidation of glucose yields 36 ATP which equates to about 32% efficiency. Where does the rest of the energy go? 22.) Where is the electron transport complexes located in the mitochondria? Speculate as to why this complex could NOT be located on the outer membrane of the mitochondria. Page 10 (Cont. # 19 AP Biology) 23.) Speculate why just the electrons are moved through the complex proteins and not the entire H+ molecule. 24.) Why does NADH create more ATP molecules than FADH2? 25.) Write out the NET equation for Cellular respiration and circle on the diagram on Page 4 of this handout the “by-products” or waste produced by this process. 26.) Speculate why the mitochondria has the “cristae” compared to the design shown to the right. 27.) Acetyl-CoA enters the Krebs cycle in what part of the Mitochondria? If we liken the mitochondria to a simple one celled organism (it having its own DNA to make proteins and enzymes), why doesn’t the mitochondria simply escape from the cell and live on its own? Date: ______________________________ Lesson Plan for Handout #19 AP Biology Objective: TLWD ability to summarize the net production of ATP and NADH through Glycolysis and Krebs, and explain how NADH is oxidized during electron transport to create a electron transfer from high energy compounds to a low energy acceptor (1/2 oxygen) and the creation of a proton gradient that will drive the ATP synthetase reaction to produce ATP when given handout #19. Content: Glycolysis, Krebs, electron transport and final electron acceptors… translating NADH to ATP. Method: Power point, white board discussion Homework: Handout #19