* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Prescribing and Quality improvement
Survey
Document related concepts
Transcript
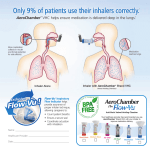
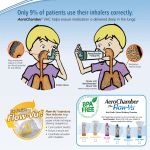
Prescribing and Quality improvement - Respiratory Step down from highest dose inhaled corticosteroids (ICS) in adult asthma Rationale High dose ICS of fluticasone 1000mcg daily (~2000mcg BDP) should be reserved for the most severe of asthmatics not controlled by other means and the evidence would expect that such a dose would only be appropriate in around 1% of all asthma patients. The numbers of patients on this dose in Somerset is significantly in excess of the expected value. This includes patients on separate inhalers e.g Flixotide or on combination Seretide or Flutiform The MeReC Bulletin Vol.22 No.03 suggests 1000 mcg/day of inhaled fluticasone has about the same effect on 8am serum cortisol levels as 10 mg/day of oral prednisolone. The MHRA advised that the prolonged use of high doses of ICS (as with the use of oral corticosteroids) carries a risk of systemic side effects, adrenal suppression, decrease in bone mineral density, cataracts and glaucoma. More recently, the MHRA warned that inhaled (and intranasal) corticosteroids can be associated with a range of psychological or behavioural effects (for example, psychomotor hyperactivity, sleep disorders, anxiety, depression and aggression). ICS have also been associated with a dose-related increased risk of both diabetes onset and progression. It is therefore a both a patient safety and cost effectiveness concern if asthma patients in Somerset are receiving excessive high dose inhaled corticosteroids. The BTS/SIGN guideline on the management of asthma recommends that reductions in ICS dose should be considered every 3 months, decreasing the dose by approximately 25-50% each time. Data suggest that this is realistic and possible without compromising patient care (see Stepping down inhaled corticosteroids in asthma: randomised controlled trial, Hawkins et al. 2003 Medications to review Flixotide Evohaler 250mcg Seretide Evohaler 250/25 Flutiform 250/10 Data for just one month (January 2015) in Somerset showed 1365 inhalers prescribed at a cost of £76,053 Main objectives To step down the Inhaled corticosteroid dose of stable asthma patients currently prescribed 1000mcg fluticasone daily. Prescribe a combination inhaler where Flixotide and a LABA are being used separately. Specific exclusion criteria Patients attended A&E or admitted for asthma in 12 months or Patients needing ITU care for asthma ever Patients coded for COPD Patients with a course of oral steroids in last 12 months Cautions Ensure that a check of inhaler technique is offered after any dose adjustments are made and that the patient is offered an alternative device which they find convenient to use Check compliance of existing treatment, particularly in patients using excessive medication exceeding a dose of 1000mcg/day of fluticasone or >12 salbutamol inhalers in last 12 months. The licensed dosage for the combination products are all two puffs bd in order to maintain the therapeutic LABA dose, therefore step-down by inhaler strength not number of puffs Evohalers are unlicensed in COPD and such patients should be switched to the licensed Accuhaler if possible re patient inhaler technique. Follow-up Agree duration of subsequent follow-up and ensure the patient is aware of how to seek help if their asthma deteriorates. Ensure patient has an Asthma management plan The MHRA advises that a steroid treatment cards should be routinely provided for people (or their parents or carers) who need prolonged treatment with high doses of ICS. The London Respiratory Network has produced a corticosteroid card that is specifically tailored for people who are using high doses of ICS greater than 1000mcg BDP equivalent. Key communication issues Studies have shown that telephone reviews are effective in improving care delivery and reducing cost .[3] Appendix 1. Sample text for letter Dear patient, Our records show that you have been taking a high dose preparation of steroid inhaler for your asthma for greater than 12 months. You may know your steroid as fluticasone, which you may be taking singly (Flixotide), or in a combination inhaler with another ingredient, such as in Seretide or Flutiform. As your condition has remained stable for the last three months, we would like to step down your strength of inhaler, as we believe it will still control your symptoms and reduce the risk of side effects of this potent medicine. Your next prescription will be for the lower strength inhaler. In the event you notice a change in your asthma symptoms, please discuss with your doctor or asthma nurse. We recommend you make every effort to attend your regular asthma check so that we can ensure your medication is exactly as you need it. We also recommend that you visit your pharmacist for an inhaler technique check if you have not had one recently. Good inhaler technique makes a big difference in how much medicine you need to take. Old medication options Flixotide 250 microgram Two puffs twice a day New medication options Flixotide 250 microgram One puff twice a day Or Seretide 250 microgram Two puffs twice a day Or Seretide 250 microgram One puff twice a day Seretide 125microgram Two puffs twice a day or Seretide 50microgram Two Puffs twice a day Or Flutiform 250 microgram Two puffs twice a day Flutiform 125 microgram Two puffs twice a day Should you have any questions about this change please contact the practice and speak to……xxx Yours faithfully, On behalf of Surgery X Biggest ICS prescribers (all strength)