* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download How to Determine the Molecular Geometry for a Compound
Survey
Document related concepts
Transcript
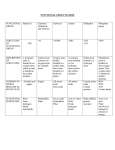
How to Determine the Molecular Geometry for a Compound 1. Draw the Lewis Dot Structure for the compound. 2. Establish the AXE designation for the Dot Structure as follows: A # of X’s # of E’s = central atom = # of BONDED atoms to the central atom = # of LONE PAIRS of electrons on the central atom The numbers of X’s and E’s are then written as subscripts. DO NOT count Lone Pair electrons on the atoms which are connected to the central atom. ONLY the lone pairs of electrons on the central atom count as E’s. 3. The total number of X’s and E’s determine the ELECTRON PAIR geometry. (There are only 5 basic electron pair geometries) NOTES: 1. If you only have 2 atoms bonded together, there is NO central atom and NO AXE designation. Since two points make a straight line, this geometry is always LINEAR. 2. If you have more than one central atom, determine the geometry on each atom separately. If the molecule is symmetrical, the geometry on each atom will be the same. If not, assign the geometry individually to each central atom. 3. If an atom has multiple bonds to a central atom, it is only counted ONCE. You count # of bonded atoms in this model, NOT # of bonds. How to Determine the Hybridization for a Compound 1. Draw the Lewis diagram for the compound. 2. The hybridization must follow the format spxdy (where x cannot be higher than 3 and y cannot be higher than 2 in this class) The total number of bonds to the central atom (X + E) must add up to the same number of letters in the hybridization. Ex.) AX3 = 3 bonds to the central atom so hybridization = sp2 (3 total letters) AX4E = 5 total bonds to central atom so hybridization = sp 3d (5 total letters) VSEPR Molecular Geometry Options!! Total Bonds (X + E) 2 (Linear) 3 (Trigonal Planar) 4 (Tetrahedral) 5 (Trigonal bipyramid) 6 (Octahedral) # bonded atoms (X) # lone pairs (E) AXE Notation Molecular Geometry Bond Angles Example Polarity 2 0 AX2 Linear 180° BeCl2 POLAR, for AX2 ONLY if X groups not identical. 3 0 AX3 Trigonal Planar 120° BCl3 POLAR, ONLY if X groups not identical. 2 1 AX2E Bent 118° NO2- YES, always polar. 4 0 AX4 Tetrahedral 109.5° CCl4 POLAR, ONLY if X groups not identical. 3 1 AX3E Trigonal Pyramid < 109.5° NH3 YES, always polar. 2 2 AX2E2 Bent < 109.5° H2O YES, always polar. 5 0 AX5 Trigonal Bipyramid 90°, 180°,120° AsCl5 POLAR, ONLY if X groups not identical. 4 1 See-saw < 90°, 180°,120° SeCl4 YES, always polar. 3 2 T-Shaped <90°,180° BrCl3 YES, always polar. 2 3 Linear <180° XeF2 POLAR, ONLY if X groups not identical. 6 0 AX6 Octahedral 90°, 180° TeBr6 POLAR, ONLY if X groups not identical. AX5E Square Pyramid < 90°,180° BrF5 YES, always polar. AX4E2 Square Planar < 90° XeF4 POLAR, ONLY if X groups not identical. 5 4 1 2 AX4E AX3E2 AX2E3