* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lithium - Learnblock
Survey
Document related concepts
Transcript
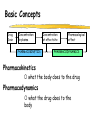
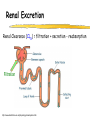
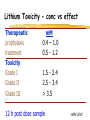
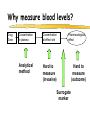
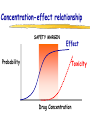
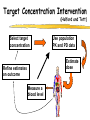
Clinical Pharmacokinetics of Lithium and Therapeutic Drug Monitoring Michael Dolton, B.Pharm (Hons) At the completion of this lecture you will understand the importance of monitoring lithium concentrations due to variability in pharmacokinetics and narrow safety margin appreciate the importance of lithium drug interactions understand the PK-PD rationale for TDM and dose adjustment Lithium First discovered in 1817 Indications Acute treatment and prevention of mania in bipolar disorder Augmentation for treatment-resistant depression Available as 250mg (Lithicarb) and 450mg SR (Quilonum SR) tablets Lithium in Bipolar disorder 1st line therapy in acute mania and prophylaxis in bipolar disorder Some second generation antipsychotics and anticonvulsants are also considered 1st line for these indications Don’t stop Lithium abruptly or treat intermittently unless toxicity present Lithium Case History Ms Ursla Down, 58 year exam supervisor, has been taking lithium for bipolar disorder. Her serum levels have ranged from 0.5 to 0.8 mM. After developing peripheral oedema and is treated with hydrochlorothiazide 100 mg mane. One week later Ursla is admitted to hospital with delirium and her serum lithium level was measured at 2.5 mM. Basic Concepts Drug Dose Concentration in plasma PHARMACOKINETICS Concentration at effect site Pharmacological effect PHARMACODYNAMICS Pharmacokinetics what the body does to the drug Pharmacodynamics what the drug does to the body Lithium Pharmacokinetics J Clin Pharmacol 1994;34:280-285. Renal Excretion Renal Clearance (CLR) = filtration + secretion - reabsorption Filtration http://www.health.bcu.ac.uk/physiology/renalsystem.htm Lithium Renal Excretion Excreted in a similar way to sodium Lithium CL = 26 mL/min GFR = 120 mL/min CLR < GFR indicates both filtration and reabsorption Not secreted Lithium CL = filtration - reabsorption Lithium unwanted effects Antagonises antidiuretic hormone in kidney producing diuresis impairs thyroid function cause weight gain hand tremor inverted T-wave of ECG Lithium Toxicity Grade I II III Manifestations tremor, nausea, vomiting, sedation, ataxia impaired consciousness, tremor, twitching semi-coma, convulsions sequelae - renal impairment, cerebral damage Lithium Toxicity - conc vs effect Therapeutic prophylaxis treatment Toxicity Grade I Grade II Grade III mM 0.4 – 1.0 0.5 - 1.2 1.5 - 2.4 2.5 - 3.4 > 3.5 12 h post dose sample AMH 2010 Lithium Toxicity - Treatment Sufficient IV fluid replacement to ensure diuresis Haemodialysis increases lithium clearance Indicated if GFR < 60mL/min, lithium > 2.5, delirium, seizures, coma, persistent clinical effects in spite of fluids Lithium concentration (mmol/L) Lithium Post-distribution sample Flat concentration-time profile after 12 h after the conventional or sustained release dose forms tablet 12 Time (h) 24 Lithium – When to monitor When staring lithium (once SS achieved) Prophylaxis: Every 3-6 months Dose changes, interacting medications started / ceased Clinical signs of toxicity, or of lack of efficacy Dehydration, or changes in salt intake Clinical status The clinical status of the patient can increase the risk of toxicity such as renal impairment or dysfunction dehydration related to fever, vomiting, diarrhoea, heavy sweating and low salt diet Drug interactions with lithium Decreases Lithium clearance Diuretics - sodium depleting (e.g. thiazide) NSAID -renal PG effects ACE inhibitors, A2RA’s Pharmacodynamic interactions effects with no change in lithium concentration anticonvulsants, SSRIs, calcium channel blockers Lithium vs. Sodium 80% of the filtered load of lithium is reabsorbed in tandem with sodium Part of Nephron Lithium Sodium Proximal Tubule ✓ ✓ Distal Tubule ✗ ✓ Loop of Henle ✗ ✓ Collecting Tubules ✗ ✓ Lithium + Thiazide diuretics Thiazide diuretics decrease sodium reabsorption in the distal tubule This creates a relative sodium deficit Leads to a compensatory increase in proximal tubule sodium and lithium reabsorption ( Lithium in blood) Lithium Case History II Lithium and the diuretic were both withdrawn. 36 h later Ms Down experiences a manic episode and lithium at the previous dose is restarted. Oedema was found to be related to lithium concentrations and safely treated with amiloride. Lithium TDM Lithium conc (mM) Confused and anorexic 1.2 mM 0.4 mM Manic episode weeks Lithium dosing diuretic Clinical Pharmacology The Right drug The Right patient The Right dose The Right time The Right Response The Right dose Do we need to measure drug concentration? For example INR or PT for warfarin serum cholesterol concentration measurements for pravastatin serum urate concentration for allopurinol blood pressure for metoprolol Why measure blood levels? Drug Dose Concentration in plasma Concentration at effect site Analytical method Hard to measure (invasive) Pharmacological effect Surrogate marker Hard to measure (outcome) What is Therapeutic Drug Monitoring? using drug concentration data as a "surrogate" of drug effect to individualise drug therapy to ensure maximum beneficial effects with minimal adverse effects. When is TDM appropriate? pharmacological response is difficult to quantify e.g. cyclosporin outcome has serious consequences concentrations used as a surrogate indicator/predictor of outcome When is TDM appropriate? drugs used as prophylactic agents (endpoint is the absence of an event) e.g. anticonvulsants, antiarrythmics, lithium drugs with a narrow therapeutic range (individualise therapy to maximise benefits and minimise risks) e.g. digoxin, anticonvulsants, cardiac antiarrhythmics, immunosuppressants When is TDM appropriate? drug clearance changes rapidly e.g. declining renal function, change in renal function post-transplant, examining drug interactions Monitor patient compliance e.g suspected poor compliance, particularly for prophylactic medicine, used in clinical trials When is TDM appropriate? investigate unexpected or unexplained effects diagnose adverse effects to define patient management especially where the adverse effects mimic the disease state e.g. cardiac arrhythmias and digoxin When is TDM appropriate? Management of overdose urine drug screen - identify potential causes of coma assign cause and decide management e.g. paracetamol - decision on administration of antidote warfarin overdose and unexplained INR Commonly monitored drugs Antibiotics Aminoglycosides, Vancomycin Antifungals Flucytosine, Itraconazole, Voriconazole, Posaconazole Anticonvulsants Phenytoin, Carbamazepine, sodium valproate Immunosuppressants Cyclosporin, Tacrolimus, Sirolimus Antiarrhythmics Digoxin, perhexiline Others - Theophylline What is a therapeutic range? An optimal concentration range for a DRUG is NOT the same as a "normal" range defined for biochemical parameters Encompasses those recorded in patients experiencing therapeutic benefit and minimal toxicity Concentration-effect relationship SAFETY MARGIN Probability Effect Toxicity Drug Concentration What is a therapeutic range? optimal range has not been defined for many drugs optimal range should only be used as a guide and should always be interpreted with the clinical assessment of the patient Cyclosporin Target range? First 3 months Kidney : 150-300 Liver : 150-300 Heart : 250-350 Heart-lung: 350-600 BMT : 100-300 maintenance 100-200 ng/ml 100-200 ng/ml 150-250 ng/ml 200-300 ng/ml 100-300 ng/ml Interpreting Drug level data accurate dose history including dose (with an assessment of compliance) and duration of therapy time of dose administration and blood sample withdrawal patient status including age, weight and organ function (e.g. renal function assessed using estimated creatinine clearance) Interpreting Drug level data biological fluid sampled (e.g. whole blood, plasma or serum) clinical status of patient (e.g. experiencing adverse effects) medication history (including drugs that may potentially interact with the drug of interest) What is the therapeutic range for THIS patient? TDM vs. TCI Therapeutic Drug Monitoring (TDM) Target Concentration Intervention (TCI) (Holford, 1996) Target Concentration Intervention (Holford and Tett) Select target concentration Use population PK and PD data Estimate dose Refine estimates on outcome Measure a blood level Dose prediction methods Empirical dose protocol Nomogram using patient covariates Bayesian Dose prediction population pharmacokinetic approach Example Nomogram - Tobramycin Massie et al, 2006 Dose prediction methods Bayesian Dose prediction uses prior knowledge of the population pharmacokinetic parameters information about the patient predict PK parameters individualize the dose computer aided relies on knowledge of population pharmacokinetics Bayesian software - TCIWorks TCIWorks – Dose prediction Role of the pharmacist in TDM Interpret relevant information Treat the patient not the number Use pharmacokinetic knowledge to make rational dose recommendations Questions? [email protected]