* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Movement Disorders and Extrapyramidal System
Survey
Document related concepts
Transcript
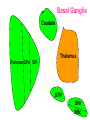
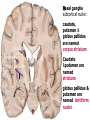
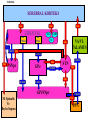
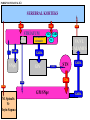
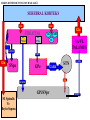
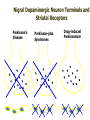
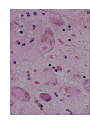
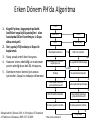
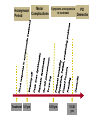
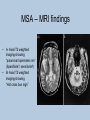
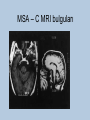
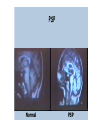
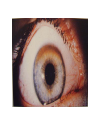
Movement Disorders and Extrapyramidal System Sibel Ertan, MD Dept. of Neurology Definition Neurologic syndromes in which there is either an excess of movement, or a paucity of voluntary and automatic movements unrelated to weakness or spasticity. Most movement disorders are associated with pathologic alterations in the basal ganglia or their connections. But disorders of the • Cerebellum or its pathways • Cerebral cortex • Thalamus • Brain stem • Spinal cord • Peripheral nerves may also cause several movement disorders. Basal Ganglia Caudate Thalamus Putamen GPe GPi STN SNr SNc Basal ganglia subcortical nuclei: caudate putamen globus pallidus caudate, putamen & globus pallidus are named corpus striatum Caudate &putamen are named striatum globus pallidus & putamen are named lentiform nuclei Definition The term extrapyramidal system, coined by British neurologist Kinnier Wilson, refers to the basal ganglia and an array of brain stem nuclei (red nucleus, reticular formation etc.) to which they are connected. Striatum (caudate+putamen) is the principle receptive structure of the basal ganglia. Globus pallidus is the principle output structure of the basal ganglia. NORMAL SEREBRAL KORTEKS Glu Glu STRİATUM D1 P Mad. DA GABA Glu Glu D2 ENK. AK Glu GABA SS Glu VA/VL TALAMUS GABA Glu SNpc GPe GABA GABA STN GABA GABA Glu Glu GPi/SNpr M. Spinalis Ve Beyin Sapına GABA Glu PPN PARKINSON HASTALIĞI SEREBRAL KORTEKS Glu Glu STRİATUM D1 P Mad. DA GABA D2/ENKEFALİN DİNORFİN AK Glu GABA SS VA/VL TALAMUS GABA SNpc GPe GABA STN GABA Glu GABA Glu GPi/SNpr M. Spinalis Ve Beyin Sapına GABA ERKEN DÖNEM HUNTINGTON HASTALIĞI SEREBRAL KORTEKS Glu Glu STRİATUM D1 P Mad. D2 ENK. Glu AK GABA SS VA/VL TALAMUS DA GABA GABA Glu SNpc GPe GABA Glu GABA GPi/SNpr M. Spinalis Ve Beyin Sapına STN GABA GABA • Diseases of the basal ganglia are associated with abnormal involuntary movements that typically occur at rest and disappear in sleep. • They are generally divided into two categories: Hyperkinetic and hypokinetic • The hyperkinetic variety is seen in such disorders as chorea, athetosis, ballism, dystonia, tremor, and tics. • The hypokinetic variety is seen largely in Parkinson’s disease and Parkinson plus syndromes • Following anatomic loci for pathology are agreed on: - Substantia nigra in Parkinson’s disease - Caudate nucleus in chorea - Subthalamic nucleus in ballism - Caudate or lentiform nucleus (especially putamen) in dystonia Hypokinetic Disorders • Parkinsonism Six cardinal features: 1. Tremor at rest 2. Rigidity 3. Bradykinesia-hypokinesia 4. Flexed posture 5. Loss of postural reflexes 6. Freezing phenomenon • Tremor, rigidity, and flexed posture are referred positive phenomena. • Bradykinesia, loss of postural reflexes, and freezing are negative phenomena. Rest Tremor • 4-5 Hz • Present in the extremities, almost always distally • Classic “pill-rolling” tremor involves the thumb and the forefinger • Rest tremor disappears with action but reemerges as the limb maintain a posture. • Rest tremor is also common in the lips, chin, and tongue • Rest tremor of the hands increases with walking • Stress worsens the tremor Rest tremor Erken evre - klinik Hastalığın premotor belirtileri: RBD Ağrı Depresyon Koku duyumunda azalma Konstipasyon Erken dönemde başka hastalıklar ile karışabilen semptomlar: <40 yaş distoni + bradikinezi Alt ekstremite bradikinezisi Distoni, rijidite, ağrı Rigidity • Increased resistance (muscle tone) to passive movement elicited when the examiner moves the patient’s limbs, neck or trunk • Equal in all directions • The underlying tremor may cause “cogwheeling” Flexed posture • Commonly begins in the arms and spreads to involve the entire body • Striatal hand • Striatal toe • Lateral tilting of the trunk is common Posture Bradykinesia • Slowness of movement, difficulty in initiating a movement, and loss of automatic movement • Hypokinesia is the reduction in amplitude of movement Bradykinesia Loss of postural reflexes • Pulltest is positive • With progress of the disease frequent fallings • The patient collapses into the chair on attempting to sit down (sitting en bloc) Freezing • Inability to perform active movements (motor block) • Often involves the legs when walking but can also involve eyelid opening,speaking and writing. Freezing Freezing The many causes of parkinsonism are divided into four categories: 1.Idiopathic (%77.7) 2. Parkinson-plus syndromes (%12.2) 3. Symptomatic (secondary) (%8.2) 4. Heredodegenerative diseases (%0.6) 1.Idiopathic (%77.7) - %10 genetic - %90 unknown 2. Parkinson-plus syndromes (%12.2) - Multisiystem atrophy - Alzheimer’s disease - Lewy body dementia - Progressive supranuclear palsy - Lytigo-bodig (ALS-dementia-Parkinsonism 3. Symptomatic (secondary) (%8.2) - Toxic - Metabolic - Drug induced - Lesions - Infections 4. Heredodegenerative diseases (%0.6) The core biochemical pathology in parkinsonism is decreased dopaminergic neurotransmission in the basal ganglia. • Degeneration of the nigrostriatal dopamine system • Degeneration of the striatum with loss of dopamine receptors • Drug induced parkinsonism as the result of blockade of dopamine receptors. Nigral Dopaminergic Neuron Terminals and Striatal Receptors Parkinson’s Disease Parkinson-plus Syndromes Drug-induced Parkinsonizm Parkinson’s disease – History 1817: James Parkinson “Shaking Palsy” 1960: Dopaminergic neuronal loss in substantia nigra 1960: Levodopa 1982: MPTP (ABD, California) (toxic substance in synthetic heroin) After 1982: Experimental models 2005: Etiopathogenesis ? Parkinson’s disease (primary parkinsonism) • Degeneration of the neuromelanin-containing neurons in the brain stem, especially in substantia nigra pars compacta and in the locus ceruleus. • Many of the surviving neurons contain eosinophilic cytoplasmic inclusions known as Lewy bodies. • By the time symptoms appear, the substantia nigra already has lost about 60% of dopaminergic neurons and the dopamine content in the striatum is about 80% less than normal. Parkinson’s disease (primary parkinsonism) • PD makes up approximately 80% of cases of parkinsonism. • Mean age at onset in both sexes is 55 years (range:20-80). • Over 60 yrs of age risk of Parkinson’s disease is %1 • Male/female = 3/2. • Prevalence 160/100.000 and incidence 20/100.000/yr. • The cause of PD is unknown. Parkinson’s Disease Unilateral onset Frequent initial sympton: unilateral upper ektremity tremor Bilateral involvement All over the course of the disease asymmetric involvement Clinical Staging of PH According to Hoehn-Yahr Scale 0- Asymptomatic 1- Unilateral involvement 2- Bilateral involvement 3- Involvement of postural reflexes, imbalance and falls Mild-moderate morbidity 4- Needs continious support 5- Bedridden Parkinson’s Disease - Symptomatology Tremor: Static postural, 3-7 Hz, (hand, arm, foot, leg, tongue, chin, lip) Bradykinesia - Hypomimia - Micrography Rijidity Disarthria (hipophoni, palilali) Gait disorder (short steps) Motor blocks (freezing) Loss of associated movements of the arms Flexed posture Parkinson’s Disease - Symptomatology Disphagia Loss of postural reflexes, falls Pain, sensory complaints Autonomic findings: Constipation, postural hypotension, sweeting, urinary incontinans, empotans Depression: ~% 50 of cases Dementia: Mild, % 20-40 of cases Diagnosis of PD Based on clinical findings and signs No radiologic “marker” No laboratory “marker” Parkinson’s disease (primary parkinsonism) Treatment • Treatment is aimed at controlling symptoms because no drug or surgicl approach unequivocally prevents progression of PD. • Treatment is lifelong. • Treatment includes pharmacotherapy, physiotherapy and surgery. Motor semptomların semptomatik tedavisi • Dopaminerjik ajanlar – – – Levodopa • Levodopa + karbidopa • Levodopa + benserazid • COMT inhibitörleri* (entakapon, tolkapon) Dopamin agonistleri • Non-ergo† – Pramipeksol – Ropinirol – Rotigotin – Piribedil – Apomorfin • Ergot deriveleri – Bromokriptin – Pergolid – Kabergolin – Dihidroergokriptin – Lisurid Selektif MAO-B‡ inhibitörleri • Selejilin • Rasajilin • Non-dopaminerjik ajanlar – – Antikolinerjik ajanlar: • Triheksifenidil • Benztropin NMDA§ antagonistleri • Amantadin * Katekol-O- metil transferaz inhibitörleri ; her zaman L-dopa ile birlikte kullanılırlar † Apomorfinin subkutan enjeksiyon formları var ve L-dopa ile ilişkili motor fluktuasyonlarda kullanılır ‡ Monoamin oksidaz tip B § N-metil-D-aspartat Schapira AHV, Olanow CW. In: Principles of Treatment in Parkinson’s Disease; 2005. PH Tedavisinde Kanıta Dayalı Etkinlik Kategori Erken Evre Monoterapi Levodopa COMT inhibitörleri √ İleri Evrede L-Dopa ile kombine MAO-B inhibitörleri Antikolinerjikler & amantadin √ ± √ (pramipeksol, ropinirol, pergolid) √ (pramipeksol, bromokriptin, kabergolin, pergolid) √(MF) ? ± Dopamin agonistleri Motor komplikasyonların tedavisi - √(MF) ? √ (D; amantadin) - (MF) Motor komplikasyonların önlenmesi - ? - (D) ? (MF) ? √ (pramipeksol, ropinirol, kabergolin) -(?) ? ? ? √ (pramipeksol, ropinirol) Görüntülemede dopaminerjik nöron kaybında yavaşlama √ ± ? 0 etkin (maksimum kanıt) olası etkinlik Etkili değil Yetersiz kanıt çalışma yok √ (pramipeksol, ropinirol, pergolid) D diskineziler MF motor fluktuasyonlar COMT “catechol-Omethyltransferase” MAO-B monoamin oksidaz B DAs dopamin agonistleri Rascol O, et al. Lancet 2002;359:1589-98. Goetz CG, et al. Mov Disord 2005;20:523-39. Fahn S, et al. N Engl J Med 2004;351:2498-508. Horstink M, et al. Eur J Neurol 2006;13:1170-85. Horstink M, et al. Eur J Neurol 2006;13:1186-202. 50 Erken Dönem PH’da Algoritma 1. Kognitif yıkımı, özgeçmişde psikotik özellikleri veya kişilik patolojileri olan hastalarda DA’leri önerilmiyor. L-Dopa daha emniyetli. 2. İleri yaşda (>70) tedaviye L-Dopa ile başlanmalı. 3. Yavaş ancak yeterli doz titrasyonu. 4. Hastanın tolere edebildiği ve maksimum yanıtın alındığı doza dek DA titrasyonu. 5. Optimum motor kontrol için zaman içerisinde L-Dopa’nın tedaviye eklenmesi Tanı Tedavi kararı HAYIR EVET Gözden geçir Özürlülüğün belirlenmesi Hafif motor özürlülük 1,2 Orta şiddette motor özürlülük, kognitif yıkım yok DA veya MAO-B inhibitörü başla 1, 2,3 DA başla Tolere edilebilir maksimum yanıta dek DA dozu arttır Ek semptomatik etki gerekli 3 4 Henüz başlanmamışsa DA başla 4 Schapira AHV, Olanow CW. In: Principles of Treatment in Parkinson’s Disease; 2005:127. © 2005 MAO-B inhibitörü eklenebilir Tolere edilebilir maksimum yanıta dek DA dozu arttır Ek tedavi gerektiren özürlülük Ek tedavi gerektiren özürlülük L-Dopa başla * Monoamin oksidaz B 5 Parkinson’s disease (primary parkinsonism) Treatment Therapeutic choices for Parkinson’s disease Medications Dopamine precursor: levodopa (LD)±carbidopa or benserazide Dopamine agonists: bromocriptine, pergolide, pramipexole, ropinirole, apomorphine, cabergoline, pribedil. Catecholamine-O-methyl transferase inhibitors:tolcapone and entacapone. Dopamine releaser, NMDA receptor antagonist: Amantadine. Monoamine oxidase type B inhibitor: selegiline. rasagiline Anticholinergics:trihexyphenidyl, benztropine, biperidene... Antihistaminics:diphenhydramine, orphenadrine, phenindamine Parkinson’s disease (primary parkinsonism) Treatment Therapeutic choices for Parkinson’s disease Surgery Ablative surgery:Thalamotomy, pallidotomy. Restorative surgery:Embryonic dopaminergic tissue transplantation Deep brain stimulation:Thalamic stimulation, pallidal stimulation, subthalamic stimulation, PPN Parkinson’s disease (primary parkinsonism) Treatment • LD is the most effective drug, BUT 75% of patients have serious complications after 5 years of LD therapy. • Younger patients, in particular, are more likely to show response fluctuations. • DOPA-SPARING STRATEGY:Other antiparkinsonian drugs should be used first to delay the introduction of LD. • Selegiline delays the need for LD therapy by an average of 9 months. Adverse events and side effects of L-Dopa Noisea, vomiting Postural hypotension Psychosis: Hallucinations delusions Late Complications of L-Dopa Motor Complications 1. “wearing-off” End of dose phenomenon (predictable) 2. “On-off” fluctuations (unpredictable) 3. Dyskinesias: - Chorea (“on” period) - Dystonia (“on” or “of” period) PD- Motor Complication L-Dopa induced motor complications “on” period Motor Honeymoon Complications Period Treatment 3-5 yrs Symptoms unresponsive to treatment 8-10 yrs 15-20 yrs PD Dementia L-Dopa response at early stage B R Thanvi & T C N Lo Postgrad. Med. J. 2004;80:452-458. L-Dopa response at mid-stage B R Thanvi & T C N Lo Postgrad. Med. J. 2004;80:452-458. L-Dopa response at late stage B R Thanvi & T C N Lo Postgrad. Med. J. 2004;80:452-458. Clinical Features Excluding Idiopathic PD • Stepwise progression of parkinsonian symptoms and history of recurrent stroke • History of recurrent head trauma • History of encephalitis • Oculogyric crisis • Neuroleptic use • Sustained remission • Unilateral involvement after 3 years of symptom-onset • Supranuclear gaze palsy Clinical Features Excluding Idiopathic PD • • • • • • • Cerebellar findings Early severe autonomic symptoms Early severe dementia Babinski sign Communicating hydrocephalus and basal ganglionic lesions Negative response to L-Dopa MPTP intoxication Multisystem Atrophy Degenerative, sporadic, rapid progressive Falls at early stages, severe dysarthria Significant symmetry Mild or absent tremor Age at onset of the disease 30 years Combination Parkinsonizm: L-Dopa response is poor/absent Cerebellar symptoms Pyramidal symptoms Autonomic dysfunction MSA - P MSA-C MSA • MRI (sensitivity %88-%60, specifisity %93- %100) – Putaminal hypointensity – In the outer border of putamen hyperintensity – Atrophy of the cerebellum, middle cerebellar peduncles, midbrain – Increased signal intensity in pons (hot cross bun) Orta hat “raphe” ve transvers pontin liflerde sinyal artışı, tegmentum, piramidal traktus ve superior serebellar pedinkül etkilenmiyor MSA – MRI findings • A- Axial T2 weighted imaging showing “putaminal hiperintens rim” (Spesifisite?, sensitivite?) • B- Axial T2 weighted imaging showing “Hot cross bun sign” MSA – C MRI bulguları Progressive supranuclear palsy (PSP) PSP PSP Normal PSP Secondary Parkinsonism - CO Intoxication Lower body parkinsonism Hyperkinetic Disorders – Tremor * Involuntary oscillations of a body part produced by alternating or synchronous contractions of reciprocally innervated muscles. * Physiological tremor These tremors are very small amplitude and are demonstrable only by means of accelerometer. Enhanced physiological tremor: medical conditions, drugs, anxiety, fear… * Essential tremor ET Typically a postural tremor (4-12 Hz) but may be accentuated by goal-directed movements. The site of involvement in most cases is the hands and it is frequently asymmetric initially. * Parkinsonian tremor Tremor at rest, at a frequency of 4-5 Hz, is the most characteristic and the most prominent type of tremor in PD, but postural and kinetic tremor are also frequently seen. Onset of the tremor is usually in one of the hands; rarely, it may begin in the legs. * Intention tremor Rhythmic involuntary oscillations that undergo exacerbation as the hand or foot approaches the target of a voluntary movement. It indicates involvement of the cerebellum or its connections. Essential tremor Goal directed tremor – Chorea (“dance”) Characterized by sudden, frequent involuntary, arrhythmic, purposeless, and quick jerks of the trunk, extremities, and head associated with facial grimaces. They are usually distal and of low amplitude. Causes of chorea are hereditary, autoimmune, vascular, metabolic, toxic, inflammatory or drug induced. – Athetosis (“without position”) Slow, writhing, continuous, wormlike movements of the distal parts of the extremities, chiefly the fingers, which show bizarre posturing. Generalized chorea Huntington disease • • • • • • • • • • • • Progressive hereditary disorder that usually appears during adult life Characterized by movement disorder (usually chorea), dementia, personality disorder Caudate nucleus and putamen are severly involved GABAergic efferents projecting to lateral globus pallidus are lost chorea Later striatal efferents to medial pallidum are lost parkinsonism, dystonia Prevalence 4-8/100.000 4p16.3, CAG-repeat disorder (N:11-34) (HD:37-86) Huntingtin protein Trinucleotide repeat is unstable in gametes More juvenil cases of HD when an individual inherits the gene from father Onset 35-40 yr (5-70) Movement disorder, personality disorder, mental deterioration (course over a period of 15 yr) – Ballism (“jump or throw”) Sudden, quick, continuous, unusually violent, and flinging in nature. Usually confined to the contralateral vascular lesion in the subthalamic nucleus. – Dystonia (“bad tone”) Twisting, slow, contorting, involuntary movement, that is somewhat sustained and often repetitive. Dystonia can involve any part of the body. Dystonia is classified as (1) focal, (2) segmental (cranial muscles (Meige syndrome), cranio-cervical dystonia, (3) multifocal (2 or more noncontiguous parts), (4) hemidystonia, (5) generalized (segmental crural + an other part of the body) Classification of Torsion Dystonia • • By age of onset – Childhood onset, 0-12 yr – Adolescent onset, 13-20 yr – Adult onset, >20 yr By distribution – Focal – Segmental – Generalized – Hemidystonia • By etiology – – – – Primary (familial, sporadic) Dystonia-plus Secondary dystonia Heredodegenerative diseases Primary Dystonias • Pure dystonia except tremor • Familial and nonfamilial sporadic types • Most primary dystonias are sporadic, adult onset focal or segmental dystonias • DYT 1 (Oppenheim dystonia) – Onset 12.5±8.2 – In %95 of patients symptoms begin in an arm or leg and the disorder spre to the neck or larynx – AD, 9q34.1, Torsin A protein – Penetrance rate 30%, 40% Dystonic storm Lingual dystonia Generalized dystonia, chorea, spasmodic dysphonia (adductor) Dystonic tremor Generalized dystonia, myoclonus Wilson disease (Hepatolenticular degeneration) • Autosomal recessive disorder with the gene being located on the long arm of chromosome 13. • The gene encodes a copper transporting P-type ATPase that is expressed in liver and kidney • Two fundamental defects: 1.reduced biliary transport of copper, 2.impaired formation of plasma ceruloplasmin • Free Cu in serum is increased • Overflow of copper from the liver produces accumulation in other organs, mainly in brain, kidney, and cornea . Wilson disease (Hepatolenticular degeneration) • In cornea, copper is deposited close to the endothelial surface of the Descement membrane (Kayser-Fleischer ring; most important diagnostic feature) • Symptoms begin between the ages of 11 and 25 years • Wilson disease is a disorder of motor function; there are no sensory symptoms and reflex alterations. • Symptoms of basal ganglia damage usually predominate but cerebellar symptoms may occasionally be in the foreground. • Tremors and rigidity are the most common early signs. • Seizures can occur at any stage of the disease. Wilson disease (Hepatolenticular degeneration) Treatment • Initial phase of the treatment (toxic copper levels are brought under control) Penicillamine Ammonium tetrathiomolybdate Triethylene tetramine dihydrohloride (trientine) • Maintenance therapy Zinc acetate Trientine + Zinc acetate