* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 340B and Your Organization
Survey
Document related concepts
Transcript
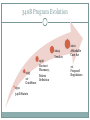
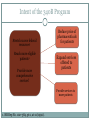
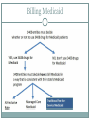
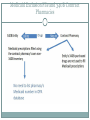
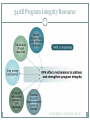
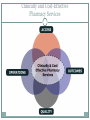
340B AND YOUR ORGANIZATION F U N G I S A I N O TA , P H D. * ANDREW WELSH ANDREW LOFURNO AIDS CARE GROUP RY A N W H I T E A L L T I T L E S M E E T I N G , WASHINGTON DC N OV E M B E R 2 7 T H - 2 9 T H , 2 0 1 2 * C O N TA C T I N F O R M A T I O N : F N O TA @ A I D S C A R E G R O U P. O R G 340B Background 340B Program Evolution 1993 1st Guidelines 1992 340B Statute 1996 Contract Pharmacy, Patient Definition 2004 Vendors 2010 Affordable Care Act 1st Proposed Regulations Creation of the 340B Program Certain safety net covered entities Outpatient drugs 340B Program Price discounts Required for all manufacturers in Medicaid Intent of the 340B Program Stretch scarce federal resources1 Reach more eligible patients1 Provide more comprehensive services1 Reduce price of pharmaceuticals for patients Expand services offered to patients Provide services to more patients 1. HR Rep No. 102–384, pt 2, at 12 (1992). Patient Definition 340B Eligible Entities * 340B eligible through Section 7101 of the Affordable Care Act Hospital Eligibility Criteria *340B eligible through Section 7101 of the Affordable Care Act Hospital Outpatient Facilities In order for outpatient facilities to become eligible for the 340B Program: The outpatient facility must be an integral part of the hospital The outpatient facility must be included as reimbursable on the covered entity’s most recently filed Medicare Cost Report To register additional outpatient facilities, complete the online Register an Outpatient Facility registration at: http://opanet.hrsa.gov/OPA/CERegister.aspx 340B Enrollment Procedure http://opanet.hrsa.gov/OPA/CERegister.aspx 340B Implementation Part 1 - Summary 1. History of 340B 2. The Intent of 340B Program 3. Who is eligible 4. Key dates 340B Prohibitions and Requirements 340B Covered Drugs 340B Prohibitions and Requirements Exclusions Diversion Duplicate Discounts Duplicate Discount on 340B Drugs Examples of Duplicate Discounts Examples of Duplicate Discounts Cont’d 1. CMS. Letter re: medication prescription drug rebates. April 22, 2010. Available at: www.ncsl.org/documents/health/42210PPACADrug_Rebate_SMD.pdf. Accessed November 22, 2011. Billing Medicaid Medicaid Exclusion File and 340B Contract Pharmacies The Medicaid Exclusion File CE Decision to Use 340B Drugs Carve-In CE Responsibilities for Avoiding Duplicate Discounts It is ultimately the responsibility of the 340B participating entity to ensure accurate reporting of Medicaid billing of any 340B drugs to OPA and the state Medicaid agency. Avoiding Duplicate Discounts Diversion Prohibition GPO Exclusion The Orphan Drug Exclusion The Orphan Drug Product Designation Database can be found at: http://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm Part 2 - Summary Determining which drugs are covered under 340B 2. Diversion / Exclusion / Duplicate discounts 3. Carving – in or Carving - out Medicaid 4. GPOs and Orphan drugs 1. OPTIMIZING YOUR 340B PROGRAM 340B Prime Vendor Program PVP Mission and Goals Improve access to affordable medications for covered entities and their patients Primary goals: Lower participants’ supply costs by expanding the current PVP portfolio of sub-340B priced products Provide covered entities with access to efficient drug distribution solutions to meet their patients’ needs Provide access to other value added products and services meeting covered entities’ unique needs Estimated Prices For Selected Public Purchasers as a Percent of AWP 0% 20% 40% 60% 80% 100.0% AWP 80.0% AMP 67.9% Medicaid (Min.) 60.5% Medicaid Net 51.7% FSS 340B 49.0% FCP 47.9% VA Contract 100% 34.6% Stephen Schondelmeyer, PRIME Institute, University of Minnesota (2001) Private Sector Pricing The 340B Price 340B Drug Pricing Program 25%–50% of the average wholesale price The 340B price is actually considered a “ceiling” price Can offer subceiling prices Drug Manufacturers Benefits of PVP to Participants Ease of enrollment and activation of pricing by wholesaler Access to 340B sub-ceiling prices for covered drugs Access to discounts on other value added outpatient products such as vaccines and diabetic supplies Participant communications Support of DSHs and HRSA grantees by funding 340B education and networking opportunities Value of PVP to Participants Savings - average sub-ceiling savings on PVP contract purchases for all participants = 16% in 2007 Diminishes the need for Independent Sub-Ceiling contracts and the resources that they require to manage Provides a “One Stop Shopping” model for outpatient pharmacy services such as 340B split-billing software Access to lowest priced vaccines in the marketplace Access to market reports to help cut formulary costs Cost Savings Analysis Summary Current Annualized Purchases = $538,576 Projected Annualized Purchases if the participant takes advantage of all categories of savings (1:1, generic exchange, and therapeutic exchange = $331,131 Annualized Savings of $205,455 Percent Savings of 38% This analysis was for a FQHC switching from a GPO Model to a 340B plus Prime Vendor Model Supplier Agreements Allendale Pharmaceutical Alliant Pharmaceuticals AMO (pending) Astra-Zeneca Pharmaceuticals Abraxis Pharmaceutical Akorn Inc. ASD (flu vaccine) Bayer Diagnostics Bedford Labs Can-am Care LLC Caraco Pharmaceutical Labs Cytogen Dabur Pharmaceuticals FFF (flu vaccine) G&W Laboratories Geritrex Corporation GlaxoSmithKline Hawthorne Pharmaceuticals, Inc Home Diagnostics Inc. Early Detect Lilly & Company Major Pharmaceuticals Medicure Morton Grove Pharm Inc. NitroMed Inc. Novartis Vaccines Novo Nordisk Okomoto USA Inc. Organon USA, Inc. Paddock Labs RD Plastics Co Inc. Rx Elite Holdings, Inc. Sandoz Pharmaceutical Teva Health Systems Total Pharmacy Supply Tri State Distribution Stratus Pharmaceuticals Trinity Biotech X-Gen Pharmaceuticals Watson Pharma Inc. Wyeth Pharmaceuticals Other Products and Services Vaccines PAP software Split billing software Auditing/overcharge recovery services Repackaging services Prescription vials/labels/printer cartridges Diabetic/TB syringes PBM services OTC diagnostic test kits HIV rapid test kits Pharmacy automation/technology Manufacturers & 340B Pricing Must provide 340B pricing if their drug(s) is covered by Medicaid Cannot sell covered drug above 340B ceiling price to covered entity Are not prohibited from selling outpatient drugs at below 340B ceiling price Prices offered covered entities are exempt from “best price” but not Non-FAMP calculation Are not required to offer sub-ceiling price to other covered entities or Medicaid Can obtain Non-FAMP pricing exemption for sub-ceiling pricing through HRSA’s 340B Prime Vendor Program Manufacturers – 340B Pricing and Medicaid Rebate Programs Medicaid and 340B entities receive prices based on either “Best Price” OR Average Manufacturer Price (AMP) – 15.1% for branded drugs Additional discounts are applied if price increases exceed the Consumer Prime Index (CPI) Generics – AMP minus 11% “Best Price” is not part of generic calculation Pricing - recalculated quarterly Discounts are upfront. No backend rebates PRIME VENDOR CONTRACTING • Contract methodology - build upon existing supplier relationships - new supplier contracting - savings vs. revenue - target high dollar/ proprietary drugs - negotiations vs. bidding on select drug classes - value added products and services CHOOSING A CONTRACT PHARMACY Negative (- ) Positive (+) Entity pays flat fee per claim Entity pays fees based on % of Stop-loss function (prevents 3rd party transmission is loss to entity) Entity does not pay fees on claim reversals Entity pays lowest of U&U, MAC, and 340B revenue or drug cost Entity does not keep Medicaid/3rd party reimbursement Vendor recruits patients to its mail order pharmacy Early cancellation fees Entity not allowed to select wholesaler Entity might end may end up purchasing partial bottles at high rates due to non-replenishment Part 3 - Summary 1. Understanding PVP 2. Expected gains from joining the PVP 3. Choosing contract pharmacies 340B POLICIES 340B Policies Guidelines Regulations (proposed) • Patient Definition* Manufacturer Civil • Contract Pharmacy* Monetary Penalties Administrative Dispute Resolution Orphan Drugs • Audits* • Dispute Resolution* • Outpatient Facilities • Duplicate Discounts 340B Guidance and Policy http://www.hrsa.gov/opa/federalregister.htm 340B Proposed Regulations Drug Delivery Contract Pharmacies 340B Usage Considerations 340B Program Support Office of Pharmacy Affairs (OPA) 340B Program Integrity Resource 340B Program Integrity Resource Functions of OPA Clinically and Cost-Effective Pharmacy Services MAKING 340B PROGRAM WORK 1. 2. 3. 4. 5. 6. Maintain updated records on OPA website. Choose your contact pharmacy wisely Conduct regular internal audits Devote adequate quality personnel for 340b Develop and update your organization’s 340B standard operating procedures (SOPs) If you deliver the medication – combine it with case management to increase adherence GETTING READY FOR AN AUDIT Have Policies and Procedures - do not create them for the purpose of the audit - detail entire process Be proactive - get information to the auditor when requested in an easily digestible format Audit Now - trace clinically significant encounters monthly - involve social workers, patient financial services, and medical records. Understand state Medicaid managed care - get to know your Medicaid office that processes claims for your entity Stand your ground with C-suite, do not be pressured into risky practices. QUESTIONS MAIN SOURCES USED OPA HRSA APEXUS – 340B PVP