* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Stoichiometry - Bruder Chemistry
Computational chemistry wikipedia , lookup
Click chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Biochemistry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Physical organic chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Process chemistry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
History of molecular theory wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
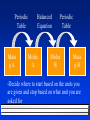
Atomic Mass Atoms are so small, it is difficult to discuss how much they weigh in grams. Use atomic mass units. an atomic mass unit (amu) is one twelth the mass of a carbon-12 atom. This gives us a basis for comparison. The decimal numbers on the table are atomic masses in amu. They are not whole numbers Because they are based on averages of atoms and of isotopes. can figure out the average atomic mass from the mass of the isotopes and their relative abundance. add up the percent as decimals times the masses of the isotopes. Examples There are two isotopes of carbon 12C with a mass of 12.00000 amu(98.892%), and 13C with a mass of 13.00335 amu (1.108%). There are two isotopes of nitrogen , one with an atomic mass of 14.0031 amu and one with a mass of 15.0001 amu. What is the percent abundance of each? The Mole The mole is a number. A very large number, but still, just a number. 6.022 x 1023 of anything is a mole A large dozen. The number of atoms in exactly 12 grams of carbon-12. The Mole Makes the numbers on the table the mass of the average atom. Representative particles The smallest pieces of a substance. For a molecular compound it is a molecule. For an ionic compound it is a formula unit. For an element it is an atom. More Stoichiometry Molar mass Mass of 1 mole of a substance. Often called molecular weight. To determine the molar mass of an element, look on the table. To determine the molar mass of a compound, add up the molar masses of the elements that make it up. Find the molar mass of CH4 Mg3P2 Ca(NO3)3 Al2(Cr2O7)3 CaSO4 · 2H2O Examples How much would 2.34 moles of carbon weigh? How many moles of magnesium in 24.31 g of Mg? How many atoms of lithium in 1.00 g of Li? How much would 3.45 x 1022 atoms of U weigh? Percent Composition Percent of each element a compound is composed of. Find the mass of each element, divide by the total mass, multiply by a 100. Easiest if you use a mole of the compound. Find the percent composition of CH4 Al2(Cr2O7)3 CaSO4 · 2H2O Working backwards From percent composition, you can determine the empirical formula. Empirical Formula the lowest ratio of atoms in a molecule. Based on mole ratios. A sample is 59.53% C, 5.38%H, 10.68%N, and 24.40%O what is its empirical formula. Pure O2 in Sample is burned completely to form CO2 and H2O CO2 is absorbed H2O is absorbed A 0.2000 gram sample of a compound (vitamin C) composed of only C, H, and O is burned completely with excess O2 . 0.2998 g of CO2 and 0.0819 g of H2O are produced. What is the empirical formula? More Stoichiometry Empirical To Molecular Formulas Empirical is lowest ratio. Molecular is actual molecule. Need Molar mass. Ratio of empirical to molar mass will tell you the molecular formula. Must be a whole number because... Example A compound is made of only sulfur and oxygen. It is 69.6% S by mass. Its molar mass is 184 g/mol. What is its formula? Chemical Equations Are sentences. Describe what happens in a chemical reaction. Reactants Products Equations should be balanced. Have the same number of each kind of atoms on both sides because ... Meaning A balanced equation can be used to describe a reaction in molecules and atoms. Not grams. Chemical reactions happen molecules at a time or dozens of molecules at a time or moles of molecules. Stoichiometry Given an amount of either starting material or product, determining the other quantities. use conversion factors from – molar mass (g - mole) – balanced equation (mole - mole) keep track. Examples How many moles is 4.56 g of CO2 ? How many grams is 9.87 moles of H2O? How many molecules in 6.8 g of CH4? 49 molecules of C6H12O6 weighs how much? Examples One way of producing O2(g) involves the decomposition of potassium chlorate into potassium chloride and oxygen gas. A 25.5 g sample of Potassium chlorate is decomposed. How many moles of O2(g) are produced? How many grams of potassium chloride? How many grams of oxygen? Examples A piece of aluminum foil 5.11 in x 3.23 in x 0.0381 in is dissolved in excess HCl(aq). How many grams of H2(g) are produced? How many grams of each reactant are needed to produce 15 grams of iron form the following reaction? Fe2O3(s) + Al(s) Fe(s) + Al2O3(s) Examples K2PtCl4(aq) + NH3(aq) Pt(NH3)2Cl2 (s)+ KCl(aq) what mass of Pt(NH3)2Cl2 can be produced from 65 g of K2PtCl4 ? How much KCl will be produced? How much from 65 grams of NH3? Gases and the Mole Many Gases of the chemicals we deal with are gases. They are difficult to weigh. Need to know how many moles of gas we have. Two things effect the volume of a gas Temperature and pressure Compare at the same temp. and pressure. Standard Temperature and Pressure 0ºC and 1 atm pressure abbreviated STP At STP 1 mole of gas occupies 22.4 L Called the molar volume Avagadro’s Hypothesis - at the same temperature and pressure equal volumes of gas have the same number of particles. Examples What is the volume of 4.59 mole of CO2 gas at STP? How many moles is 5.67 L of O2 at STP? What is the volume of 8.8g of CH4 gas at STP? Density of a gas D = m /V for a gas the units will be g / L We can determine the density of any gas at STP if we know its formula. To find the density we need the mass and the volume. If you assume you have 1 mole than the mass is the molar mass (PT) At STP the volume is 22.4 L. Examples Find the density of CO2 at STP. Find the density of CH4 at STP. Given The other way the density, we can find the molar mass of the gas. Again, pretend you have a mole at STP, so V = 22.4 L. m = D x V m is the mass of 1 mole, since you have 22.4 L of the stuff. What is the molar mass of a gas with a density of 1.964 g/L? 2.86 g/L? Stoichiometry Greek for “measuring elements” The calculations of quantities in chemical reactions based on a balanced equation. We can interpret balanced chemical equations several ways. Look at it differently 2H2 + O2 2H2O 2 dozen molecules of hydrogen and 1 dozen molecules of oxygen form 2 dozen molecules of water. 2 x (6.02 x 1023) molecules of hydrogen and 1 x (6.02 x 1023) molecules of oxygen form 2 x (6.02 x 1023) molecules of water. 2 moles of hydrogen and 1 mole of oxygen form 2 moles of water. Mole to mole conversions 2 Al2O3 Al + 3O2 every time we use 2 moles of Al2O3 we make 3 moles of O2 2 moles Al2O3 3 mole O2 or 3 mole O2 2 moles Al2O3 Mole to Mole conversions How many moles of O2 are produced when 3.34 moles of Al2O3 decompose? 2 Al2O3 Al + 3O2 3.34 moles 3 mole O2 = 5.01 moles O2 Al2O3 2 moles Al O 2 3 Your Turn 2C2H2 + 5 O2 4CO2 + 2 H2O If 3.84 moles of C2H2 are burned, how many moles of O2 are needed? How many moles of C2H2 are needed to produce 8.95 mole of H2O? If 2.47 moles of C2H2 are burned, how many moles of CO2 are formed? Periodic Table Mass gA •Decide Balanced Equation Moles A Periodic Table Moles B Mass gB where to start based on the units you are given and stop based on what unit you are asked for For example... If 10.1 g of Fe are added to a solution of Copper (II) Sulfate, how much solid copper would form? Fe + CuSO4 Fe2(SO4)3 + Cu 2Fe + 3CuSO4 Fe2(SO4)3 + 3Cu 10.1 g Fe 1 mol Fe 3 mol Cu 63.55 g Cu 55.85 g Fe 2 mol Fe 1 mol Cu 17.3 g Cu = More Examples To make silicon for computer chips they use this reaction SiCl4 + 2Mg 2MgCl2 + Si How many moles of Mg are needed to make 9.3 g of Si? 3.74 mol of Mg would make how many moles of Si? How many grams of MgCl2 are produced along with 9.3 g of silicon? For Example The U. S. Space Shuttle boosters use this reaction 3 Al(s) + 3 NH4ClO4 Al2O3 + AlCl3 + 3 NO + 6H2O How much Al must be used to react with 652 g of NH4ClO4 ? How much water is produced? How much AlCl3? Gases and Reactions We can also change Liters of a gas to moles At STP 0ºC and 1 atmosphere pressure At STP 22.4 L of a gas = 1 mole If 6.45 moles of water are decomposed, how many liters of oxygen will be produced at STP? For Example If 6.45 grams of water are decomposed, how many liters of oxygen will be produced at STP? H2O H2 + O2 2H2O 2H2 + O2 6.45 g H2O 1 mol H2O 1 mol O2 22.4 L O2 18.02 g H2O 2 mol H2O 1 mol O2 Your Turn How many liters of CO2 at STP will be produced from the complete combustion of 23.2 g C4H10 ? What volume of oxygen will be required? Yield How much you get from an chemical reaction Limiting Reagent If you are given one dozen loaves of bread, a gallon of mustard and three pieces of salami, how many salami sandwiches can you make? The limiting reagent is the reactant you run out of first. The excess reagent is the one you have left over. The limiting reagent determines how much product you can make Limiting Reagent Reactant that determines the amount of product formed. The one you run out of first. Makes the least product. Book shows you a ratio method. It works. So does mine Limiting reagent To determine the limiting reagent requires that you do two stoichiometry problems. Figure out how much product each reactant makes. The one that makes the least is the limiting reagent. How do you find out? Do two stoichiometry problems. The one that makes the least product is the limiting reagent. For example Copper reacts with sulfur to form copper ( I ) sulfide. If 10.6 g of copper reacts with 3.83 g S how much product will be formed? If 10.6 g of copper reacts with 3.83 g S. How many grams of product will be formed? Cu is 2Cu + S Cu2S Limiting 1 mol Cu2S 159.16 g Cu2S 1 mol Cu Reagent 10.6 g Cu 63.55g Cu 2 mol Cu 1 mol Cu2S = 13.3 g Cu2S 1 mol S 3.83 g S 32.06g S 1 mol Cu2S 159.16 g Cu2S 1 mol S 1 mol Cu2S = 19.0 g Cu2S Example Ammonia is produced by the following reaction N2 + H2 NH3 What mass of ammonia can be produced from a mixture of 100. g N2 and 500. g H2 ? How much unreacted material remains? How much excess reagent? Use the limiting reagent to find out how much excess reagent you used Subtract that from the amount of excess you started with Excess Reagent The reactant you don’t run out of. The amount of stuff you make is the yield. The theoretical yield is the amount you would make if everything went perfect. The actual yield is what you make in the lab. Your turn Mg(s) +2 HCl(g) MgCl2(s) +H2(g) If 10.1 mol of magnesium and 4.87 mol of HCl gas are reacted, how many moles of gas will be produced? How much excess reagent remains? Your Turn II If 10.3 g of aluminum are reacted with 51.7 g of CuSO4 how much copper will be produced? How much excess reagent will remain? Percent Yield % yield = Actual x 100% Theoretical % yield = what you got x 100% what you could have got Yield The amount of product made in a chemical reaction. There are three types Actual yield- what you get in the lab when the chemicals are mixed Theoretical yield- what the balanced equation tells you you should make. Percent yield = Actual x 100 % Theoretical Example 6.78 g of copper is produced when 3.92 g of Al are reacted with excess copper (II) sulfate. 2Al + 3 CuSO4 Al2(SO4)3 + 3Cu What is the actual yield? What is the theoretical yield? What is the percent yield? If you had started with 9.73 g of Al, how much copper would you expect? Examples Aluminum burns in bromine producing aluminum bromide. In a laboratory 6.0 g of aluminum reacts with excess bromine. 50.3 g of aluminum bromide are produced. What are the three types of yield. Examples Years of experience have proven that the percent yield for the following reaction is 74.3% Hg + Br2 HgBr2 If 10.0 g of Hg and 9.00 g of Br2 are reacted, how much HgBr2 will be produced? If the reaction did go to completion, how much excess reagent would be left? Examples Commercial brass is an alloy of Cu and Zn. It reacts with HCl by the following reaction Zn(s) + 2HCl(aq) ZnCl2 (aq) + H2(g) Cu does not react. When 0.5065 g of brass is reacted with excess HCl, 0.0985 g of ZnCl2 are eventually isolated. What is the composition of the brass?