* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download INFECTIONS IN TRANSPLANTATION
Survey
Document related concepts
Transmission (medicine) wikipedia , lookup
Urinary tract infection wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Globalization and disease wikipedia , lookup
Viral phylodynamics wikipedia , lookup
Germ theory of disease wikipedia , lookup
Marburg virus disease wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Schistosomiasis wikipedia , lookup
Hepatitis C wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Infection control wikipedia , lookup
Neonatal infection wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Transcript
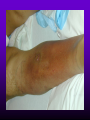
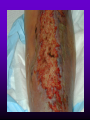
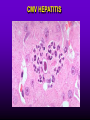
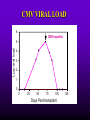
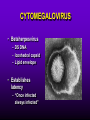
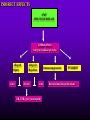
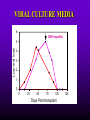
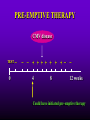
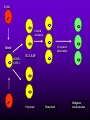
INFECTIONS IN SOLID ORGAN TRANSPLANT RECIPIENTS DR. ATUL HUMAR INFECTIOUS DISEASES / MULTI-ORGAN TRANSPLANTATION OBJECTIVES • To review the concept of compromised host • To gain an understanding of the common infections after transplant • To gain further understanding into herpesvirus infections after transplant – CMV – EBV Definition • Compromised host – Patient lacks resistance to infection due to a deficiency in defense mechanisms against microbial invasion and/or disease – Inherited or acquired PATHOGENESIS Microbe Host Defense Mechanisms Inoculum or Organisms Virulence Latency DISEASE DETERMINANTS HOST DEFENSE MECHANISMS • Intact skin and mucous membranes – Disrupted due to trauma, burns, ulceration, IV catheters, surgery • Types of infection – Wound infections, burn sepsis, diabetic foot infection, line sepsis • Usual organisms – Bacteria – environmental, endogenous – Fungi – environmental, nosocomial HOST DEFENSE MECHANISMS • Physical removal / clearance of micro-organisms – Respiratory muco-ciliary clearance – Peristalsis and dynamics of hollow viscus (gut, bile ducts, ureter, fallopian tube) – Maybe abnormal due to underlying disease, surgery, smoking etc. • Intact sphincters/valves • Types of infection – Pneumonia, urosepsis, biliary sepsis • Usual organisms – Bacteria – environmental, endogenous HOST DEFENSE MECHANISMS • Endogenous microflora – Oropharyngeal, gut, skin, vagina – Important for preventing colonization with disease causing organisms (competitive) – Antibiotics remove natural flora – E.g. C. difficile colitis • Chemical antimicrobial agents – Gastric acidity, cutaneous fatty acids HOST DEFENSE MECHANISMS • Inflammatory response – Number (mass) and function of circulating and tissue phagocytic cells – Neutrophils, monocytes, macrophages, spleen • Humoral Mediators – Complement, fibronectin HOST DEFENSE MECHANISMS • Specific Immune response – T-lymphocytes • CD4+, CD*+ (helper, cytotoxic) • Number, function – B-lymphocytes • Make antibodies • IgG, IgA Common problems Host Defect: Inflammatory response Common microbes Neutropenia (<0.5) Gram negative bacilli, Staph, Candida, Aspergillus Splenectomy S. Pneumonia, H. influenza, N. Meningitis Common problems Host Defect: Complement Common microbes Early (C3, C5) S. Aureus, S. Pneumonia, gram negative bacilli Late (C6,7,8) Neisseria species Common problems Host Defect: Immune response Common microbes T- Lymphocyte e.g. HIV, organ transplant Numerous microbes B-Lymphocyte S. Pneumonia, H. influenza, Giardia INFECTION: BASIC PRINCIPLES • Inflammatory response attenuated by immunosup. • may abolish typical signs/symptoms • decreased sensitivity of serological, radiological tests • Efffects of established infection may be devastating • Treatment may have more toxicities • Rifampin - decrease CsA • Erythromycin, azoles increase CsA • Synergistic nephrotoxicity - aminoglycosides, AmB, septra, cipro, vancomycin, pentamidine INFECTIONS IN TRANSPLANTATION Three main determinants of the risk of infection in transplant recipients • Infections related to technical / surgical problems TECHNICAL COMPLICATIONS • Liver - biliary tree - leaks, strictures • Lung - bronchial anastomosis necrosis, dehiscence ; mediastinal fluid collection • Kidney - uroterocystostomy - leak, urinoma • Pancreas - duodenum-bladder; duodenum-bowel: anastomotic leaks, abscess INFECTIONS IN TRANSPLANTATION Major determinants of the risk of infection The net state of Immunosuppression Epidemiological exposures NET STATE OF IMMUNOSUPPRESSION • Immunosuppressive therapy: dose, duration, temporal sequence - ‘area under the curve’ • Underlying immune deficiency • Mucocutaneous barrier integrity: intubation, drains, catheters, central lines • Devitalized tissue, fluid collection • Neutropenia, lymphopenia NET STATE OF IMMUNOSUPPRESSION • Metabolic conditions • Uremia • Malnutrition • Diabetes • Viral infection: Immune modulation • Cytomegalovirus • Epstein-Barr virus • Hepatitis B, C, HIV EPIDEMIOLOGICAL EXPOSURES Community – – – – Community acquired pneumonia pathogens Environmental fungi Enteric bacterial pathogens (salmonella) TB, zoonosis, HIV, hepatitis viruses Nosocomial – MRSA,VRE – Pseudomonas, MDR gram negatives – Aspergillus CASE PRESENTATION • 61 y.o. male heart transplant 1991 • Stable immunosuppression x years – cylosporin, prednisone • 3 week history of progressive leg cellulitis, fever unresponsive to antibiotics • Intermittent confusion TIMETABLE: 0-1 MONTH • Infections usual to post-op patients – nosocomial pneumonia, wound, line sepsis, UTI • Key factors: nature of the operation, technical skill • Lung, heart, liver at highest risk – longer intubation, ICU stay, lines, catheters • Most OI’s (eg. PCP) absent in the first month – Exceptions – HSV, HHV6, Candida, Aspergillus TIMETABLE: 0-1 MONTH • Also may see – Infection transmitted with the allograft: eg. lung transplant with pneumonia or a donor bacteremia which seeds the vascular anastamosis – Pre-existing infection within the recipient made worse by the transplant TIMETABLE OF INFECTION One to 6 months post-Tx – Maximal period of immunosuppression – Effect of sustained immunosuppression or ‘area under the curve’ – Opportunistic infections in the absence of excessive epidemiological hazard TIMETABLE - 1 TO 6 MONTHS VIRAL – CMV, EBV, VZV, HHV-6, Adenovirus, Influenza, RSV BACTERIAL – Nocardia, Legionella, Listeria, TB FUNGAL – PCP, Aspergillus, Cryptococcus, endemic mycosis PARASITIC – Toxoplasma, Strongyloides TIMETABLE - > 6 MONTHS GROUP 1: Good graft function, minimal immunosuppression – Community acquired pneumonia, UTI, OI based on intense exposure GROUP 2: Recurrent or chronic rejection, high level immunosuppression, chronic viral replication – Continued risk of opportunistic infections CASE PRESENTATION • 55 year old female OLTx for PSC • Acute rejection: Steroid resistant requiring OKT3 for 10 days (pre-emptive ganciclovir) • Neoral, prednisone, MMF • 2 months later presents with fever, malaise, elevated transaminases CMV HEPATITIS CMV VIRAL LOAD 6 CMV hepatitis Log viral load 5 4 3 2 1 0 0 25 50 75 100 Days Post-transplant 125 CYTOMEGALOVIRUS • Betaherpesvirus – DS DNA – Icoshedral capsid – Lipid envelope • Establishes latency – “Once infected always infected” CLINCAL MANIFESTATIONS DIRECT EFFECTS – Asymptomatic viral shedding – Acute viral syndrome – Pneumonitis: BMT, Lung Transplant – Infection of allograft: hepatitis, pneumonitis, nephritis, myocarditis, pancreatitis – Infection of native tissue: GI, CNS, retina INDIRECT EFFECTS CMV INFECTION/DISEASE Cellular effects: Antigen/cytokineexpression Allograft Injury acute Allograft Rejection chronic Immunosuppression acute OB, VBD, graft vasculopathy PTLD-EBV Bacterial and fungal infections ANTILYMPHOCYTE ANTIBODIES OTHER HERPES VIRUSES REJECTION SEPSIS/ SURGERY INFLAMMATION (CYTOKINES, NF-B) LATENT CMV INFECTION CMV: THE ROLE OF CYTOKINES • TNF-a has been shown to stimulate the CMV-IE gene enhancer/promotor region in a dose-dependent manner leading to CMV reactivation • CMV has direct effects on cytokines: CMV-IE gene products shown to increase IL-6 and IL-8 gene expression • This has been shown to enhance neutrophil trans-endothelial migration • Cytokine mediated PMN recruitment may enhance CMV dissemination CYTOKINE LEVELS AND CMV DISEASE Humar et al. J Infect Dis 1999; 179: 484 Steroids CsA MMF Increasing viral load CMV INFECTION CMV DISEASE MULTIVARIATE ANALYSIS OF RISK FACTORS FOR CMV DISEASE Factor CMV serostatus D-/R+ D+/R-* D+/R+ Peak viral load (prior to disease) Steroid boluses P value P = 0.57 P = 0.0001 P = 0.35 Induction immunosuppression Double vs. triple therapy P= 0.11 Humar et al. Transplantation 2000 OR (95% CI) OR = 1.40 (1.111.49) † HHV-6 AND TRANSPLANTATION • Cytopathic lymphotrophic virus : roseola infantum • Seroprevalence almost universal by age 2-3 • Post-transplant: implicated as a cause of febrile illness, hepatitis, pneumonitis and other infections. • Rates of reactivation estimated from 14 - 82 % • Its main effect post-transplant may be immunomodulatory including an interaction with CMV HHV-6 AND TRANSPLANTATION • Infection of T-cells results in down-regulation of IL-2 mRNA and protein synthesis, and a reduction in mitogen-driven proliferative responses resulting in a cell mediated immune defect • HHV-6 infection results in cytokine dysregulation; induction of TNF-a and other immunomodulatory cytokines • Interactions among herpesviruses may be more direct, including specific binding via glycoproteins resulting in cellular co-infections and facilitating viral spread VIRAL CULTURE MEDIA 6 CMV hepatitis Log viral load 5 4 3 2 1 0 0 25 50 75 100 Days Post-transplant 125 HERPESVIRUS INTERACTIONS • Serial Quantitative HHV-6 in 200 liver transplant recipients • Serial Quantitative CMV PCR • Direct effects and Indirect effects of viral replication on development of graft rejection and opportunistic infection were assessed HHV-6 RESULTS • HHV-6 infection occurred in 28% (56/200) patients (defined as VL > 2 logs) • peak VL occurred at a median of 35 days (mean 44.1 days; range 8-177) • Symptomatic disease occurred in only 2/200 patients (1%) and presented as fever and pancytopenia HHV-6 AND CMV Outcome Multivariate model OR (95% CI) CMV disease n=32 HHV-6 infection Antilymphocyte globulin Steroid boluses Immunosuppression 3.27 (1.43-7.51) 3.13 (1.31-7.55) 1.00 (0.84-1.19) 2.15 (0.85-7.50) P-value 0.005 0.01 0.99 0.094 CMV PREVENTION • Universal prophylaxis: anti-viral therapy to all ‘atrisk’ patients • Pre-emptive therapy: anti-viral therapy to subgroups of ‘at-risk’ patients usually based on further diagnostic tests aimed at identifying early viral reactivation PRE-EMPTIVE THERAPY CMV disease TEST 0 _ _ _ + + + + + + + 4 8 _ _ 12 weeks Could have initiated pre-emptive therapy CMV IN LIVER TRANSPLANT RECIPIENTS PRE-TRANSPLANT: Donor and recipient CMV serology POST-TRANSPLANT: • D+/R+, D-/R+ – Week 2-12: Every clinic visit: CMV antigenemia CMV quantitative PCR • D+/R-: – Ganciclovir prophylaxis 12 weeks Bloodwork at week 12, 14, 16, 18. CMV antigenemia and quantitative PCR testing STUDY PROTOCOL: CMV IN LIVER TRANSPLANT RECIPIENTS OUTCOME: • CMV disease defined according to biopsy evidence; viral syndrome based on specific clinical criteria ANALYSIS: • Predictive value for antigenemia and PCR – Positive: >0 cells/slide; >400 copies/ml – Sensitivity, specificity, PPV and NPV for different cut-off points – Multivariate logistic regression for predictors of CMV disease RESULTS • CMV disease: 21/97 ( 21.7%) patients; mean 60 days posttransplant • PCR: sensitivity of 100%, specificity 47.4%, PPV 34.4 % and NPV 100% for prediction of CMV disease • Antigenemia: 95.2%, 55.3%, 37.0% and 97.7 %. • The optimal cut-off for PCR in the range of 2000-5000 copies/ml (sensitivity 85.7%, specificity 86.8%, PPV 64.3%, NPV 95.7) • The optimal cut-off for antigenemia was in the range of 6 positive cells/slide. ROC CURVE FOR CMV QUANTITATIVE PCR >1000 >0 1.00 >2000 >5000 0.75 Sensitivity >7000 >12000 0.50 >15000 >20000 0.25 0.00 0.00 0.25 0.50 1- Specificity 0.75 1.00 PREEMPTIVE THERAPY • CMV antigenemia or CMV quantitative PCR useful for predicting the development of CMV disease • Either of these tests could be employed in a preemptive strategy using optimal cut-offs • The CMV viral load is the most important determinant for the development of CMV disease RESPONSE TO THERAPY 6 Log viral load CMV hepatitis 5 4 3 2 1 0 0 25 50 75 100 125 Days Post-transplant 8 weeks after treatment relapsed with fever, Recurrent CMV disease RESPONSE TO THERAPY • Virologic response to therapy assessed in 52 patients with CMV disease treated with ganciclovir • Viral loads done at regular intervals after starting treatment • Genotypic resistance testing • Clinical response to treatment : Relapsing disease occurred in 24% of patients Humar et al. JID 2002 VIRAL LOAD KINETICS y=y0eax Viral load (log10 copies/ml) 6 5 4 3 2 1 0 0 10 Time20 (days) 30 40 FIGURE 1 Patient 1 Patient 2 6 5 5 4 4 3 3 2 2 T1/2 = 1.3d 1 T1/2= 1.6d 1 0 0 0 5 10 15 20 25 0 5 Time (days) 10 20 25 Time (days) Patient 3 6 15 Patient 4 6 5 5 4 4 3 3 2 T1/2 = 4.5d 2 1 T1/2 = 6.2d 1 0 0 10 20 Time (days) 30 40 0 0 10 20 30 40 50 60 Time (days) Humar et al. JID 2002 Factor Viral load at onset (log10 copies/mL) Mean S.D. (median; range) Time to clear CMV (days) Mean S.D (median; range) Viral load half-life (days) Mean S.D (median; range) Number who cleared viral load n (%) No relapse (n=40) Relapse (n=12) P-value 4.82 4.94 (4.67; 2.865.66) 4.73 4.67 P=NS (4.65; 3.795.22) 17.2 9.3 (16; 5-43) 33.8 11.6 (30; 21-47) 3.17 3.35 (1.94; 0.7218.2) 8.83 6.30 P=0.001 (6.61; 1.2319.7) 39 (97.5%) 5 (41.7%) P=0.002 P<0.001 CONCLUSIONS • Different people have different rates of response to antiviral therapy • Early phase kinetics are predictive of relapsing disease. • Differential response likely combination of – Host factors - CTL, immunosuppression – Viral Factors – genotype, immune evasion genes EBV AND PTLD DEFINITION • An abnormal proliferation of B-cells driven by EBV – May be polyclonal or monoclonal – (occasional tumors are T-cell, NK cell) PTLD Viral Infection Tumor EPSTEIN-BARR VIRUS • Lytic infection – ~100 genes expressed, lysis of B-cell • Latent infection – – – – < 10 genes expressed LMP 1,2, EBNA 1,2,3, EBER, BCRF, BHRF, BARF Evades host immune response Latent gene products drive B-cell proliferation Lytic Growth advantage Cytogenetic abnormality latent BCRF-1 LMP-1 IL-1,6,10 Polyclonal Monoclonal Malignant transformation CASE PRESENTATION • 34 y.o. male 2 years post-kidney transplant • On Neoral, Prednisone and Immuran • Fever, sore throat, and multiple subcutaneous nodules INVESTIGATIONS • EBV Viral load > 1000 copies / 106 PBL • Biopsy – Aggressive, undifferentiated monoclonal PTLD, EBV positive • Withdrawal of MMF, treatment with IV ganciclovir Log viral load (-o-) 6 5 Ganciclovir 4 3 2 1 0 0 1 2 3 TIME (months) 4 RISK FACTORS FOR PTLD • EBV D+/R– 1-5% incidence in R+ vs. 20-30% in R- • Intensity of Immunosuppression • Type of transplant – Small bowel > lung > heart > liver, kidney • Herpesvirus interactions EBV SEMIQUANTATIVE PCR Timing of Test PTLD (log/106) No PTLD (log/106) P-value Prior to PTLD 2.9(1.5) 1.4(1.5) 0.005 PTLD Diagnosis 3.1(1.2) 1.4(1.5) < 0.001 Peak value (12 months post-transplant) 3.4(0.5) 1.8(1.5) < 0.001 EBV PROPHYLAXIS STUDY • Multicentre RCT in EBV D+/Rtransplant recipients – Group 1: Ganciclovir + CMVIG – Group 2: Ganciclovir + placebo • EBV viral loads taken at regular intervals post-transplant EBV PROPHYLAXIS STUDY • Viral load data was analyzed from 28 (20 pediatric and 8 adult) patients (15 cytogam and 13 placebo). • Transplant types were liver (n=11), kidney (n=10), lung (n=6), and pancreas (n=1). • During the first 6 months post-transplant, detectable viremia occurred in 9/13 (69.2%) placebo patients and 10/15 (66.7%) cytogam patients SUMMARY • Reactivation of herpesviruses post-transplant are due to a complex interaction of multiple factors • Viral infections likely produce multiple direct and indirect effects on the post-transplant course of these patients • Efforts to minimize the impact of these infections should lead to improvement in graft outcomes