* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 5 Thermochemistry
Adiabatic process wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermodynamic system wikipedia , lookup
History of thermodynamics wikipedia , lookup
Internal energy wikipedia , lookup
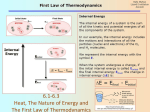
Thermochemistry Thermodynamics = study of energy and its transformations Thermochemistry = study of chemical reactions involving changes in heat energy Thermochemistry © 2009, Prentice-Hall, Inc. Energy Energy = the ability to do work or transfer heat energy. Work = energy used to cause an object with mass to move (w = f x d) Heat = energy used to cause the temperature of an object to increase Thermochemistry © 2009, Prentice-Hall, Inc. Major Types of Energy Potential energy = energy an object possesses by virtue of its position or chemical composition. Kinetic energy = energy an object possesses by virtue of its motion. Thermochemistry © 2009, Prentice-Hall, Inc. Kinetic Energy 1 KE = m v 2 2 m = mass in kilograms (kg) v = velocity in meters per second (m/s) KE = kinetic energy in joules (J) 1 Joule = 1 kg-m2/s2 A mass of 2 kg moving at a speed of one meter per second possesses a kinetic energy of 1 Joule. Thermochemistry © 2009, Prentice-Hall, Inc. Potential Energy PE = m g h m = mass in kilograms (kg) g = acceleration due to gravity (9.8 m/s2) h = height (m) PE = potential energy in joules (J) 1 Joule = 1 kg-m2/s2 Thermochemistry © 2009, Prentice-Hall, Inc. Units of Energy • The SI unit of energy is the joule (J). • An older, non-SI unit is still in widespread use: the calorie (cal). 1 cal = 4.184 J • 1000 calories = one nutritional Calorie Thermochemistry © 2009, Prentice-Hall, Inc. First Law of Thermodynamics • Energy is neither created nor destroyed, but it can undergo a transformation from one type to another. (Law of Conservation of Energy) • The total energy of the universe is a constant. • The energy lost by a system must equal the energy gained by its surroundings, and vice Thermochemistry versa. © 2009, Prentice-Hall, Inc. System and Surroundings System = the molecules we want to study (here, the hydrogen and oxygen molecules). Surroundings = everything else (here, the cylinder and piston). Thermochemistry © 2009, Prentice-Hall, Inc. Internal Energy The internal energy of a system is the sum of all kinetic and potential energies of all components of the system; we call it E. E = Efinal − Einitial (It’s a state function) • If E is positive, the system absorbed energy from the surroundings. • If E is negative, the system released energy Thermochemistry to the surroundings. © 2009, Prentice-Hall, Inc. E = q + w • When energy is exchanged between the system and the surroundings, it is exchanged as either heat (q) or work (w). • That is, E = q + w. Thermochemistry © 2009, Prentice-Hall, Inc. q, w, and their signs + q = system gains or takes in heat - q = system loses or gives off heat + w = work is done on the system by the surroundings (piston pushed in) - w = work is done by the system on its Thermochemistry surroundings (piston moves out) © 2009, Prentice-Hall, Inc. Example As hydrogen and oxygen gas are ignited in a cylinder, the system loses 550 J of heat to its surroundings. The expanding gases move a pistion to do 240 J of work on its surroundings. E for system = ? Answer: E = q + w E = (-550 J) + (-240 J) E = - 790 J What does it mean? The system gave off 790 J of energy to its surroundings Thermochemistry © 2009, Prentice-Hall, Inc. Enthalpy & H • The symbol for enthalpy is H. • Enthalpy is the internal energy plus the product of pressure and volume: H = E + PV • At constant pressure: H = E = q • So at constant pressure, H = heat lost or gained by the system. Thermochemistry © 2009, Prentice-Hall, Inc. Recall: Endothermic • When heat is absorbed (taken in) by the system from the surroundings, the process is endothermic. H = Hfinal − Hinitial H = Hproducts − Hreactants H = positive value for endothermic Thermochemistry © 2009, Prentice-Hall, Inc. Recall: Exothermic When heat is released (given off) by the system into the surroundings, the process is exothermic. H = Hfinal − Hinitial H = Hproducts − Hreactants H = negative value for exothermic Thermochemistry © 2009, Prentice-Hall, Inc.