* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Severe Acute Respiratory Syndrome
Compartmental models in epidemiology wikipedia , lookup
Public health genomics wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Canine parvovirus wikipedia , lookup
Canine distemper wikipedia , lookup
Henipavirus wikipedia , lookup
Marburg virus disease wikipedia , lookup
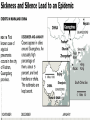
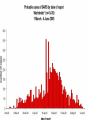
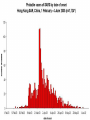
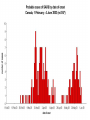
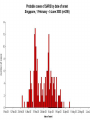
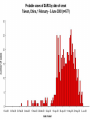
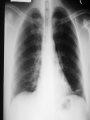
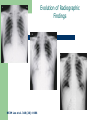
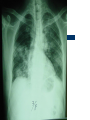
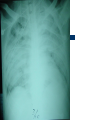
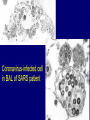
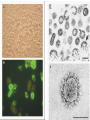
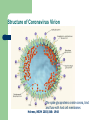
Severe Acute Respiratory Syndrome (SARS) David S. Stephens MD Age of Aquarius “One can think of the middle of the 20th century as one of the most important social revolutions in history- the elimination of the infectious disease as a significant factor in social life” Sir Frank MacFarland Burnet 1962, 1960 Nobel Laureate for Medicine “Infectious Diseases will be eliminated as a major threat to human health” US Surgeon General 1967 Microbial Evolution Ignored historical and ecological data that emergence and reemergence of infections have been common place in nature throughout evolution – – – – Plague - Hepatitis C - Diphtheria Anthrax - Dengue - Helicobacter HIV - EBOLA - Hantavirus Lyme - Legionnaire’s Disease - West Nile Factors in Emergence and Reemergence of Infections Microbial Mutation and Horizontal Recombination – – – Rapid generation time and high copy number 3.8 billion years of microbial evolution and diversity The vast majority of microorganisms remain uncultured and unknown Urbanization and Land Use Globalization and Population Growth Environmental and Social Changes Severe Acute Respiratory Syndrome (SARS) Emergence Clinical Features Pathogenesis Transmission and Infection Control Treatment The Future Severe Acute Respiratory Syndrome (SARS) Atypical pneumonia/ARDS caused by a newly identified coronavirus First recognized in Hanoi, Vietnam on February 26th, 2003 by Dr Carlo Urbani. As of June 6th, WHO had received reports of 8404 cases of probable SARS from China, Hong Kong Special Administrative Region of China, Canada, Vietnam, Singapore, Thailand, United States and 22 other countries. Thus far 779 people have died and 5937 have recovered (11.6 % mortality). PATIENT A Physician from Guangdong province China Onset of symptoms on February 15, 2003 Visit to relatives in Hong Kong 21 February Stayed in Hotel M in Room 911 Admitted to Hong Kong Hospital 22 February and died the next day 12 patients in Hotel M, 2 family members and 4 Health Care Workers infected Patient B 47 YO Asian-American textile businessman stayed on 9th floor at Hotel M on 21 February On February 23rd traveled to Hanoi and became ill on February 26th was admitted to a hospital in Hanoi with high fever, dry cough, myalgias and mild sore throat. Over the next 4 days he developed increasing respiratory difficulties, thrombocytopenia and then ARDS. He was transferred to a hospital in Hong Kong but died on March 12th, 2003 On March 5th, 2003, seven healthcare workers who had cared for the patient B in Hanoi also became ill… Spread from Hotel M MMWR 2003; 52(12):241 Canada Guangdong Province, China F,G A F,G 18 HCW 11 close contacts A Hotel M Hong Kong A Hong Kong SAR 95 HCW H,J H,J K B Ireland K 0 HCW I, L,M C,D,E I,L,M >100 close contacts B C,D,E Vietnam Singapore 37 HCW 34 HCW 21 close contacts 37 close contacts United States 1 HCW SARS Cases Worldwide Reported to WHO as of June 6, 2003 Europe: 8 countries (38) Canada (219) U.S. (68) China (5329) Hong Kong (1750) SA (2) Vietnam (63) Thailand (8) Taiwan (676) Singapore (206) Australia&NZ (6) Total: 8404 cases; 779 deaths (~10%case fatality) Donnelly, Lancet.com May 7, 2003 Masked shop owner in Amoy Gardens complex photo by Christian Keenan Timeline of SARS Cases in Canada NEJM 2003;348;1995 SARS cases by date of hospitalization, Singapore*—Feb 25–Mar 22, 2003 7 No. of cases 6 5 Tertiary(6) Secondary(16) Primary(19) Index(3) 4 3 2 1 * Data provided by WHO Date of onset ar 19 -M ar 17 -M ar 15 -M ar 13 -M ar 11 -M ar 9M ar 7M ar 5M ar 3M b ar 1M 27 -F e 25 -F e b 0 68 Reported Cases of Probable SARS, United States through June 5, 2003 2 3 1 9 2 MA 2 1* 21* 1* 3 1 2 1 1 3* 2 2 2 1 1* 1 1 1 1 4 HI 2 CT 3 NJ 1* SARS - Clinical Features Asymptomatic or mild respiratory illness Moderate respiratory illness – Temperature of >100.4º F (>38º C)*, and – One or more clinical findings of respiratory illness (e.g., cough, shortness of breath, difficulty breathing, or hypoxia). Severe respiratory illness – Fever and respiratory symptoms as above and radiographic evidence of pneumonia, or respiratory distress syndrome, or autopsy findings consistent with pneumonia or respiratory distress syndrome without an identifiable cause SARS – Clinical presentation Incubation period 2-7(10) days Patients abruptly develop high fever (>38° C), chills and rigors and other and flu-like symptoms including headache, myalgias followed in 3-7 days by symptoms of respiratory illness including cough, shortness of breath and hypoxia. Radiographic findings can be initially normal or those of patchy pneumonia which may progress to bilateral infiltrates and ARDS. Symptoms Commonly Reported By Patients with SARS1-5 Symptom Fever Cough Dyspnea Chills/Rigor Myalgias Headache Diarrhea 1. Range (%) 100 57-100 20-100 73-90 20-83 20-70 10-67 Unpublished data, CDC. 2. Poutanen SM, et al. NEJM 3/31/03. 3. Tsang KW, et al. NEJM. 3/31/03 4. Peiris JSM, et al. Lancet 4/8/03 5. Lee N. et al NEJM 4/7/03 SARS – Diagnostic evaluation Chest x-ray O2 saturation Blood cultures Sputum Gram stain and culture Testing for bacterial and viral respiratory pathogens: – – – Influenza A and B and RSV Legionella, C. pneumoniae, mycoplasma, etc Save clinical specimens for possible additional testing – Respiratory, Blood, Serum – Acute and convalescent sera (>21 days from symptom onset) SARS – Laboratory findings Hypoxemia Leucopenia with lymphopenia Thrombocytopenia Transaminase elevation (ALT/AST 1-3 times upper limit of normal) CPK elevation LDH elevation Common Clinical Findings in Patients with SARS1-5 Finding Examination Rales Hypoxia Laboratory Leukopenia Lymphopenia Low platelet Increased ALT Increased LDH Increased CPK Range (%) 38-90 60-83 17-34 54-89 17-45 23-78 70-94 26-56 1. Unpublished data, CDC. 2. Booth CM, et al. JAMA 5/6/03. 3. Tsang KW, et al. NEJM. 3/31/03 4. Peiris JSM, et al. Lancet 4/8/03 5. Lee N. et al NEJM 4/7/03 Radiographic Features of SARS Infiltrates present on chest radiographs in > 80% of cases Infiltrates – initially focal in 50-75% – interstitial – Most progress to involve multiple lobes, bilateral involvement NEJM Lee et al. 348 (20): 1986 Evolution of Radiographic Findings NEJM Lee et al. 348 (20): 1986 NEJM, Ksiazek et al. 2003;348: 1953 Coronaviruses Single Strand RNA, nonsegmented, enveloped, ~31,000 bps Order: Nidovirales Family: Coronaviridae Torovirus and Coronavirus :Grp I, Grp II, Grp III 229E and OC43 in humans cause ~1/3 of common colds, reinfections common May remain viable for several hours after drying on surfaces Relative Size of Coronaviruses Compared to Other Microbes NY Times 4/27/03 Structure of Coronavirus Virion - The spike glycoproteins create corona, bind and fuse with host cell membranes Holmes, NEJM 2003;348: 1948 Coronavirus Biology and Disease: General Themes Recurrent / repeated infections Prolonged or persistent virus shedding Direct viral and immune mediated disease “loose” species barrier: cross infections (natural or experimental) M Denison Vanderbilt Coronavirus Molecular Biology: General Themes High mutation rate: 104 per template per replication (3 changes per genome) RNA-RNA homologous recombination Result: rapid adaptation, recovery from deleterious mutations, mechanisms to acquire and regain virulence. M Denison, Vanderbilt Coronaviruses, Hosts and Diseases Antigenic Group Virus I II III Host Respiratory HCoV-229E TGEV PRCoV FIPV FECoV CCoV human pig pig cat cat dog X HCoV-OC43 MHV RCoV HEV BCoV human mouse rat pig cattle X X X X X IBV TCoV chicken turkey X Enteric Other X X X X X X ?? X X X X X X X X CDC release attachment entry translation mRNA synthesis replication maturation assembly nucleus M Denison, Vanderbilt Genome Organization A E 1a 1b 5,000 1 10,000 15,000 S 20,000 M 25,000 29,727 nt N 30,000 B 20,001 25,000 30,000 X1 E X3 M S X2 N X4 X5 8.3 kb RNA 2 4.5 kb RNA 3 3.4 kb RNA 4 RNA 5 2.5 kb 1.7 kb RNA 6 - Replicases (1a/1b) & structural genes (S,E,M,N) - Multiple small genes (X1-X5)-these vary in number, location, and sequence in different coronaviruses CDC SARS-CoV is similar in general genome organization to other coronaviruses SARS-CoV is genetically distinct from other known coronaviruses –Structural proteins are < 40% identical –Replicase proteins are < 70% identical –SARS-CoV nsps are not homologous to known proteins Specific RT-PCR assays will allow the rapid and sensitive detection of the virus, aiding in control CDC Enterovirus Reference Laboratory - Distinct from other known coronaviruses - Neither a mutant nor recombinant - Previously unknown, probably from a nonhuman host, has acquired the ability to infect humans. Evidence that Urbani Coronavirus is the Etiology of SARS Culture of novel coronavirus from SARS patients in multiple sites worldwide Identical Sequence EM’s from BAL and lung showing coronavirus PCR finding novel coronavirus nucleic acid Antibody response specific to novel coronavirus, sera from other human coronaviruses show no reaction Infection re-produced in primate animal model CIVET CAT Nocturnal Animal Related To Mongoose Delicacy in Southern China NY Times 4/27/03 SARS ASSOCIATED NOVEL CORONAVIRUS Previously unrecognized coronavirus Genetically distinct from human (229E)or known animal coronaviruses Phylogeny: between bovine coronavirus and avian infectious bronchitis virus Animal reservoir, civets other animals? Diagnosis Confirmed Case Detection of antibody to SARS-CoV in specimens obtained during acute illness or >21 days after onset, or Detection of SARS-CoV RNA by RT-PCR confirmed by a second PCR assay, or Isolation of SARS-CoV Probable Case Suspected Case RT-PCR Urbani SARS Coronavirus Real Time PCR (Orf 1B) Sputum 108 molecules/ml (DAY 9) Plasma 100 molecules/ml (Day 9) Feces + (Day 25) Drosten et al. NEJM: April 10, 2003 Viral Shedding in Nasopharyngeal Secretions Peiris J, et al. Lancet.com 5/9/03 SARS-CoV Antibody Assays Very low or absent antibody in controls and persons without acute SARS Interpretation of results – – – Single positive sera indicative of acute infection Acute sera may be positive as early as 6 days after onset of symptoms Convalescent sera should be positive by 21-28 days after onset Transmission Animal to Human Human to Human – – – – – Large Respiratory Droplet Nuclei Contact with objects contaminated with secretions Airborne?, aerosol –generating procedures Fecal Oral? Super spreaders (sheaders?) Other Probable SARS cases by reported source of infection,* --- Singapore February 25--April 30, 2003 MMWR 2003;52:405 MMWR 2003;52:405 SARS – Travel History Thus far US patients have: – – – A history of travel to Hong Kong, Taiwan, People's Republic of China, Toronto, Singapore, Hanoi within ten days of symptom onset. Close contact with persons with respiratory illness having the above travel history. (Close contact includes having cared for, having lived with, or having had direct contact with respiratory secretions and body fluids of a person with SARS). Community Transmission, not in US SARS- Infection Control Most HCW transmission occurred without proper barrier precaution Early recognition and isolation is key – Heightened suspicion – Triage procedures Transmission may occur during the early symptomatic phase, ? before both fever and respiratory symptoms develop SARS – Infection Control Put a surgical mask on the patient and place on respiratory (negative pressure room and use of N-95 respirator masks for anyone entering the room) and contact precautions (gown, gloves, goggles for contact with the patient). Hand hygiene In some settings ninety percent of the most recent cases have been among healthcare workers. Hospital epidemiology and infectious diseases should be notified immediately. A thermal sensor checks passenger temperatures at an airport in Guangdong province NY Times 4/27/03 Selling masks near Vancouver airport AP photo - Chuck Stoody Sars-Infection Control Isolation – Hand hygiene – Contact Precautions (gloves, gown) – Eye protection – Environmental cleaning – Airborne Precautions (N-95 respirator, negative pressure) SARS RISKS FOR DISEASE SEVERITY CO-INFECTIONS TREATMENT – – Antiviral Immune modulation RISK FACTORS FOR PROGRESSION OF SARS AGE >40, >>50 years Underlying Disease (Diabetes, Heart Disease, Lung Disease, Smoking?) Hypoxia at Presentation <95%,<<90% O2 Saturation Progressive Pulmonary Infiltrates Elevated LDH >350 U/L, CPK >500U/L, Decreased Platelet Count <150,000 cu3/ml Co-Infections ? Paramyxovirus Metapneumovirus Rhinovirus Chlamydia pneumoniae SARS - Treatment A variety of antiviral (ribavirin, neuraminidase inhibitors, etc), antimicrobials (levoquin, ceftriaxone, azithromycin, doxycycline, etc) as well as corticosteroids have been used. Immunoglobulin preparation from convalescent patients SARS and RIBAVIRIN No in vitro activity or ribavirin, at 100 ug/ml or greater concentrations, against SARS coronavirus Huggins et al USAMRID INTERFERON Intranasal interferon αA administered to people prior to infection with coronavirus 229E reduced the severity of illness and viral replication [Higgins PG, 1983]. No studies have evaluated systemic interferon. Huggins et al USAMRID OTHER SARS ANTIVIRALS? Other compounds that have shown activity against selected coronavirus strains by in vitro or in vivo animal studies include hygromycin B, monolaurin, 7-thia-8-oxoguanosine, cyclopentenylcytosine, and cystatin A and D [Macintyre G, 1991; Hierholzer JC, 1982; Higgins PG, 1991; Smee DF, 1990; Smee DF, 1990; DeClercq F, 1991; Collins AR, 1998; Collins AR, 1991]. None of these compounds have formulations that would be available for use soon and further evaluation would be needed regarding their specific activity against coronaviruses and potential toxicity Immune Modulation Ribravirin?? Macrolides?? Steroids – – – Broncholitis Obliterans Organizing Pneumonia (BOOP) Acute Interstitial Pneumonia ARDS Gamma Globulin Convalescent Immune Globulin SARS Treatment No control data regarding therapy No specific therapy has been shown to be effective No in vitro activity of ribravirin against SARS coronavirus Interferon beta may have activity Immunomodulation of uncertain benefit Cover for typical and atypical causes of pneumonia SARS- The Present Transmissible respiratory infection with no effective vaccine or drugs Recognition and Interruption of transmission is key – Identify and isolate infected persons Has potentially to become endemic Aggressive and sustained infection control Voluntary isolation and quarantine are inconvenient, but have the potential to save lives and they will work to control spread SARS- The Future Rapid Diagnostic Test, Sensitive RT-PCR Antiviral Therapy, cysteine proteinase inhibitors? Identification of “Super” spreaders, transmission routes, period of infectiousness Spectrum of Disease: influenza, co-infections Vaccine Understanding why species “jump occurred”