* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Verdana 30 pt
Heat capacity wikipedia , lookup
Equipartition theorem wikipedia , lookup
Temperature wikipedia , lookup
Calorimetry wikipedia , lookup
Non-equilibrium thermodynamics wikipedia , lookup
Heat transfer wikipedia , lookup
Thermal conduction wikipedia , lookup
Conservation of energy wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Heat transfer physics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
Internal energy wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Adiabatic process wikipedia , lookup
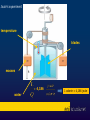
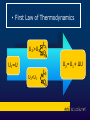
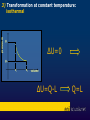
Liceo Scientifico Isaac Newton Physics course The first law of thermodynamics Professor Massimo Patrone Read by Cinzia Cetraro Historical notes • • • • Until 1600 combustion Industrial Revolution Newton Heat: source of mechanical work • Carnot and Joule • Thermodynamics James Joule (1818-1889) From energy to heat energy: Joule's experiment • The prevailing view of scientists was that the flow of heat between two bodies at different temperatures consisted in the transition from the warmer to the colder of a fluid, called caloric. Until 1700 Benjamin Thompson James Joule • Called into question the model based on caloric fluid and understood the nature of heat energy. He suggested that the friction generated by the work of a body on another body produces heat. • Confirmed Thompson ‘s intuition around 1843 showing that when mechanical work is transformed entirely into heat, the relationship between work, measured in joules, and heat, expressed in calories, remains constant in any process. Joule's experiment temperature blades masses h = 4,186 water 1 calorie = 4,186 joule The First Law of Thermodynamics A thermodynamic system is any device that exchanges heat and work with the external environment The First Law of Thermodynamics is a special formulation of the energy conservation principle, applied to thermodynamic systems. It express the changes in the internal energy of a system when it performs work or it exchanges heat with the environment. Internal Energy A state function The internal energy is molecular motion Measured in joules Sum between kinetic energy and potential energy Internal energy U of a system is the sum of the kinetic energy of molecules and the different types of potential energy associated with the bonds between the atoms that compose the system kinetic energy of molecules potential energy of bond internal energy U • First Law of Thermodynamics U2>U1 U1=U U2<U1 Ls Qa Lf Qc U2=U1+ ∆U ΔU = Q–L Quasi-static processes Piston or elementary machine To understand the processes that involve real thermodynamic systems, which are rather complex, we refer to the simple model of heat engine, consisting of a cylinder with a sliding piston and containing a perfect gas. The perfect gas • Is the only substance of which we can describe the behavior with relatively simple and accurate laws, based on measures of volume, pressure and temperature, said state quantities; these, we add the internal energy U of an ideal gas, which is all kinetic and depends only on the temperature. U = U(T) Reversible and irreversible transformations The real thermodynamic processes give rise in the PV plane, to a graphic representation which is not possible to describe mathematically because for a given volume of fluid, the pressure has different values. So they are irreversible. In a very slow process, there are a succession off equilibrium states. This transformation is called quasi-static. The quasi-static transformations can be played in reverse. 1) isobaric 4)adiabatic transformations in the perfect gas 3)isothermal 2)isochoric ΔX pressure 1) Transformation at constant pressure: isobaric pi=pf Vi Vf volume pressure 2) Transformation at constant volume: isochoric pi L=0 pf ΔU=Q-L=Q Vi=Vf volume pressure 3) Transformation at constant temperature: isothermal pi ΔU=0 pf Vi Vf volume ΔU=Q-L Q=L 4) Transformation without heat exchange: adiabatic Q=0,L<0 ΔU=Q-L ΔU=-L >0 ΔT>0 Q=0,L>0 ΔU=Q-L ΔU=-L <0 ΔT<0 heat engines Thomas Savery (1650-1715) Thomas Newcomen (1663-1729) James Watt (1736-1819) Charles Parsons (1854-1931) Thermodynamic cycles Sadi Carnot (17961832) ΔU= 0 0=Q-L Q=L A C C C The Carnot engine Heat engine human body the end Special thanks to prof. Cinzia Cetraro for linguistic supervision