* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 5 Thermochemistry
Rutherford backscattering spectrometry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Thermodynamics wikipedia , lookup
Solar air conditioning wikipedia , lookup
Transition state theory wikipedia , lookup
Heat transfer wikipedia , lookup
Internal energy wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
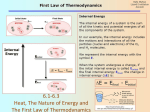
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 Thermochemistry John D. Bookstaver St. Charles Community College Edited by Debbie Amuso Thermochemistry © 2009, Prentice-Hall, Inc. Thermochemistry Thermodynamics = study of energy and its transformations Thermochemistry = study of chemical reactions involving changes in heat energy Thermochemistry © 2009, Prentice-Hall, Inc. Energy Energy = the ability to do work or transfer heat energy. Work = energy used to cause an object with mass to move (w = f x d) Heat = energy used to cause the temperature of an object to increase Thermochemistry © 2009, Prentice-Hall, Inc. Major Types of Energy Potential energy = energy an object possesses by virtue of its position or chemical composition. Kinetic energy = energy an object possesses by virtue of its motion. Thermochemistry © 2009, Prentice-Hall, Inc. Kinetic Energy 1 KE = m v 2 2 m = mass in kilograms (kg) v = velocity in meters per second (m/s) KE = kinetic energy in joules (J) 1 Joule = 1 kg-m2/s2 A mass of 2 kg moving at a speed of one meter per second possesses a kinetic energy of 1 Joule. Thermochemistry © 2009, Prentice-Hall, Inc. Potential Energy PE = m g h m = mass in kilograms (kg) g = acceleration due to gravity (9.8 m/s2) h = height (m) PE = potential energy in joules (J) 1 Joule = 1 kg-m2/s2 Thermochemistry © 2009, Prentice-Hall, Inc. Units of Energy • The SI unit of energy is the joule (J). • An older, non-SI unit is still in widespread use: the calorie (cal). 1 cal = 4.184 J • 1000 calories = one nutritional Calorie Thermochemistry © 2009, Prentice-Hall, Inc. First Law of Thermodynamics • Energy is neither created nor destroyed, but it can undergo a transformation from one type to another. (Law of Conservation of Energy) • The total energy of the universe is a constant. • The energy lost by a system must equal the energy gained by its surroundings, and vice Thermochemistry versa. © 2009, Prentice-Hall, Inc. System and Surroundings System = the molecules we want to study (here, the hydrogen and oxygen molecules). Surroundings = everything else (here, the cylinder and piston). Thermochemistry © 2009, Prentice-Hall, Inc. Internal Energy The internal energy of a system is the sum of all kinetic and potential energies of all components of the system; we call it E. E = Efinal − Einitial (It’s a state function) • If E is positive, the system absorbed energy from the surroundings. • If E is negative, the system released energy Thermochemistry to the surroundings. © 2009, Prentice-Hall, Inc. E = q + w • When energy is exchanged between the system and the surroundings, it is exchanged as either heat (q) or work (w). • That is, E = q + w. Thermochemistry © 2009, Prentice-Hall, Inc. q, w, and their signs + q = system gains or takes in heat - q = system loses or gives off heat + w = work is done on the system by the surroundings (piston pushed in) - w = work is done by the system on its Thermochemistry surroundings (piston moves out) © 2009, Prentice-Hall, Inc. Example As hydrogen and oxygen gas are ignited in a cylinder, the system loses 550 J of heat to its surroundings. The expanding gases move a pistion to do 240 J of work on its surroundings. E for system = ? Answer: E = q + w E = (-550 J) + (-240 J) E = - 790 J What does it mean? The system gave off 790 J of energy to its surroundings Thermochemistry © 2009, Prentice-Hall, Inc. Enthalpy & H • The symbol for enthalpy is H. • Enthalpy is the internal energy plus the product of pressure and volume: H = E + PV • At constant pressure: H = E = q • So at constant pressure, H = heat lost or gained by the system. Thermochemistry © 2009, Prentice-Hall, Inc. Recall: Endothermic • When heat is absorbed (taken in) by the system from the surroundings, the process is endothermic. H = Hfinal − Hinitial H = Hproducts − Hreactants H = positive value for endothermic Thermochemistry © 2009, Prentice-Hall, Inc. Recall: Exothermic When heat is released (given off) by the system into the surroundings, the process is exothermic. H = Hfinal − Hinitial H = Hproducts − Hreactants H = negative value for exothermic Thermochemistry © 2009, Prentice-Hall, Inc. Enthalpy of Reaction 1. This quantity, H, is called the enthalpy of reaction, or the heat of reaction. 2. Enthalpy is an extensive property. 3. Reverse Rx Negate H value. 4. Phase (state) matters. Thermochemistry © 2009, Prentice-Hall, Inc. Enthalpies of Formation An enthalpy of formation, Hf, is defined as the enthalpy change for the reaction in which a compound is made from its constituent elements in their elemental forms. Example: 2 Al (s) + 3 O2 (g) 2 Al2O3 (s) Thermochemistry Hf = -3340 kJ © 2009, Prentice-Hall, Inc. Standard Enthalpies of Formation Standard enthalpies of formation, Hf°, is defined as the enthalpy change for the reaction in which one mole of a compound is made from its constituent elements in their standard states at 25o C (298 K) and 1.00 atm pressure. Example: 2 Al (s) + 3/2 O2 (g) Al2O3 (s) Hf°= - 1670 kJ/mol Thermochemistry © 2009, Prentice-Hall, Inc. Calculation of H H = n Hf°products – m Hf° reactants where n and m are the coefficients. Hf°Values are listed in Appendix C of your Textbook. Thermochemistry © 2009, Prentice-Hall, Inc. Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) H = [3(-393.5 kJ) + 4(-285.8 kJ)] – [1(-103.85 kJ) + 5(0 kJ)] = [(-1180.5 kJ) + (-1143.2 kJ)] – [(-103.85 kJ) + (0 kJ)] = (-2323.7 kJ) – (-103.85 kJ) = -2219.9 kJ H = n Hf products – m Hf° reactants Thermochemistry © 2009, Prentice-Hall, Inc. Calorimetry is Another Way to Measure H values We measure H through calorimetry, which measures heat flow. But first, some definitions. Thermochemistry © 2009, Prentice-Hall, Inc. Heat Capacity The amount of energy (joules) required to raise the temperature of a substance by 1 K (1C) is its heat capacity. Units are J/K or J/ C q = C * T or C = q / T q = heat in Joules C = heat capacity in J/K T = change in temperature Thermochemistry © 2009, Prentice-Hall, Inc. Specific Heat Specific heat capacity (or simply specific heat) is the amount of energy (joules) required to raise the temperature of 1 g of a substance by 1 K. Units are J/g-K Thermochemistry © 2009, Prentice-Hall, Inc. Molar Heat Capacity • Molar heat capacity is the amount of energy (joules) required to raise the temperature of 1 mole of a substance by 1 K. Units are J/mol-K Also helpful: # mol = mass/gfm Thermochemistry © 2009, Prentice-Hall, Inc. Use Units What is the molar heat capacity of CH4 (g) if its specific heat capacity is 2.20 J/g-K? Answer: 2.20 J x 16.0 g = g–K 1 mol 35.2 J/mol-K Thermochemistry © 2009, Prentice-Hall, Inc. Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the H for the system by measuring the heat change for the water in the calorimeter. q = m sh T Thermochemistry © 2009, Prentice-Hall, Inc. Calorimetry q = m * sh * T q = Joules of heat m = total mass in calorimeter (If a solution, use m = D * V to obtain the mass) Water has a density of 1.00 g/mL Most water solutions have a density of 1.02 g/mL sh = specific heat (for water, 4.18 J/g-K) T = Tf - Ti Thermochemistry © 2009, Prentice-Hall, Inc. Calorimetry Σq=0 In other words, the heat released (or absorbed) by the reaction of interest = the sum of the heat gained (or released) by the resulting solution & calorimeter. qcalorimeter + qreaction+ qresulting solution = 0 If we assume qcalorimeter is negligible the equation reduces to the following: qreaction = - qsolution = -m*sh*ΔT where T = Tf - Ti and m = D * V H = qreaction /# moles Thermochemistry © 2009, Prentice-Hall, Inc. Specific Heat of a Metal How to determine specific heat of a metal: 1) Mass metal 2) Put metal in HOT water & measure initial temp of hot metal 3) Measure temp of 100.0 mL (100.0 g) of COLD water 4) Put hot metal in cold water 5) Record temp of water with metal in it (that temp is the final temp for both the metal & water) 6) Calculate the sh of the metal (m * sh * T)metal = (m * 4.18 * T)water Thermochemistry © 2009, Prentice-Hall, Inc. Bomb Calorimetry • Reactions can be carried out in a sealed “bomb” such as this one. • The heat absorbed (or released) by the water is a very good approximation of the enthalpy change for the reaction. Thermochemistry © 2009, Prentice-Hall, Inc. Bomb Calorimetry • Because the volume in the bomb calorimeter is constant, what is measured is really the change in internal energy, E, not H. • For most reactions, the difference is very small. Thermochemistry © 2009, Prentice-Hall, Inc. H via Heat Capacity q = C * T q = heat in Joules C = heat capacity in J/K T = change in temperature where T = Tf – Ti H = q / # moles Thermochemistry © 2009, Prentice-Hall, Inc. Stoichiometric Determination of H Given the H for a reaction, you can use mole ratios to determine H values of different sample sizes. See examples. Thermochemistry © 2009, Prentice-Hall, Inc. Sample Problem #1 H2 (g) + I2 (g) 2 HI (g) H = +53.0 kJ How many joules of heat are required to form 5 moles of HI (g)? 5 mol HI 2 mol HI = __x___ +53.0 kJ x = +133 kJ Thermochemistry © 2009, Prentice-Hall, Inc. Sample Problem #2 H2 (g) + I2 (g) 2 HI (g) H = +53.0 kJ How many moles of HI (g) are formed by the expenditure of 235 kJ of heat energy? __x___ 2 mol HI = +235 kJ +53.0 kJ x = 8.87 mol HI Thermochemistry © 2009, Prentice-Hall, Inc. Sample Problem #3 H2 (g) + I2 (g) 2 HI (g) H = +53.0 kJ How many joules of heat are required to form 109 grams of HI? # mol HI = mass/gfm # mol HI = 109 g / 128 g/mol = 0.852 mol HI 0.852 mol HI 2 mol HI = __x___ +53.0 kJ Thermochemistry x = + 22.6 kJ © 2009, Prentice-Hall, Inc. Hess’s Law H is well known for many reactions, and it is inconvenient to measure H for every reaction in which we are interested. However, we can estimate H using published H values and the properties of enthalpy. Thermochemistry © 2009, Prentice-Hall, Inc. Hess’s Law Hess’s law states that “If a reaction is carried out in a series of steps, H for the overall reaction will be equal to the sum of the enthalpy changes for the individual steps.” H = H1 + H2 + H3 Thermochemistry © 2009, Prentice-Hall, Inc. Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • Imagine this as occurring in three steps: C3H8 (g) 3 C (graphite) + 4 H2 (g) 3 C (graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) Thermochemistry © 2009, Prentice-Hall, Inc. Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • Imagine this as occurring in three steps: C3H8 (g) 3 C (graphite) + 4 H2 (g) 3 C (graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) Thermochemistry © 2009, Prentice-Hall, Inc. Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • Imagine this as occurring in three steps: C3H8 (g) 3 C (graphite) + 4 H2 (g) 3 C (graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) Thermochemistry © 2009, Prentice-Hall, Inc. Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • The sum of these equations is: C3H8 (g) 3 C (graphite) + 4 H2 (g) 3 C (graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) Thermochemistry © 2009, Prentice-Hall, Inc. Energy in Foods Most of the fuel in the food we eat comes from carbohydrates and fats. Thermochemistry © 2009, Prentice-Hall, Inc. Energy in Fuels The vast majority of the energy consumed in this country comes from fossil fuels. Thermochemistry © 2009, Prentice-Hall, Inc. Summary: Ways to Calculate H H = n Hf°products – m Hf° reactants Using Hfo values from Appendix C Or By Using a Calorimeter qreaction = - qsolution = - m*sh*ΔT where T = Tf - Ti and m = D * V H = qreaction /# moles Thermochemistry © 2009, Prentice-Hall, Inc. Summary: Ways to Calculate H Using Heat Capacity q = C * T q = heat in Joules C = heat capacity in J/K T = change in temperature where T = Tf – Ti Thermochemistry H = q / # moles © 2009, Prentice-Hall, Inc. Summary: Ways to Calculate H Hess’s Law H = H1 + H2 + H3 Or By Stoichiometric Determination Given the H for a reaction, you can use mole ratios to determine H values of different sample sizes. Thermochemistry © 2009, Prentice-Hall, Inc.