* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download flhDC
Survey
Document related concepts
Transcript
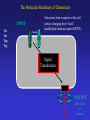
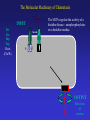
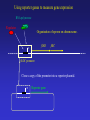
Chemotaxis and Motility in E. coli Examples of Biochemical and Genetic Networks • Background • Chemotaxis- signal transduction network • Flagella gene expression – genetic network Dictyostelium- adventures in multicellularity Julie Andreotti – Oscillations in a Biochemical Network Bacterial Chemotaxis Flagellated bacteria “swim” using a reversible rotary motor linked by a flexible coupling (the hook) to a thin helical propeller (the flagellar filament). The motor derives its energy from protons driven into the cell by chemical gradients. The direction of the motor rotation depends in part on signals generated by sensory systems, of which the best studied analyzes chemical stimuli. Chemotaxis - is the directed movement of cells towards an “attractant” or away from a “repellent”. • For a series of QuickTime movies showing swimming bacteria with fluorescently stained flagella see: http://www.rowland.org/bacteria/movies.html • For a review of bacterial motility see Berg, H.C. "Motile behavior of bacteria". Physics Today, 53(1), 24-29 (2000). (http://www.aip.org/pt/jan00/berg.htm) A photomicrograph of three cells showing the flagella filaments. Each filament forms an extend helix several cell lengths long. The filament is attached to the cell surface through a flexible ‘universal joint’ called the hook. Each filament is rotated by a reversible rotary motor, the direction of the motor is regulated in response to changing environmental conditions. The E. coli Flagellar Motor- a true rotary motor Rotationally averaged reconstruction of electron micrographs of purified hook-basal bodies. The rings seen in the image and labeled in the schematic diagram (right) are the L ring, P ring, MS ring, and C ring. (Digital print courtesy of David DeRosier, Brandeis University.) Tumble (CW) Smooth Swimming or Run (CCW) Chemotactic Behavior of Free Swimming Bacteria No Gradient Increasing attractant Increasing repellent A ‘Soft Agar’ Chemotaxis Plate A mixture of growth media and a low concentration of agar are mixed in a Petri plate. The agar concentration is not high enough to solidify the media but sufficient to prevent mixing by convection. The agar forms a mesh like network making water filled channels that the bacteria can swim through. A ‘Soft Agar’ Chemotaxis Plate Bacteria are added to the center of the plate and allowed to grow. A ‘Soft Agar’ Chemotaxis Plate As the bacteria grow to higher densities, they generate a gradient of attractant as they consume it in the media. Attractant Concentration cells cells A ‘Soft Agar’ Chemotaxis Plate The bacteria swim up the gradients of attractants to form ‘chemotactic rings’ . This is a ‘macroscopic’ behavior. The chemotactic ring is the result of the ‘averaged” behavior of a population of cells. Each cell within the population behaves independently and they exhibit significant cell to cell variability (individuality). A ‘Soft Agar’ Chemotaxis Plate ‘Serine’ ring ‘Aspartate’ ring Each ‘ring’ consists of tens of millions of cells. The cells outside the rings are still chemotactic but are just not ‘experiencing’ a chemical gradient. Serine and aspartate are equally effective “attractants”, but in this assay the attractant gradient is generated by growth of the bacteria and serine is preferentially consumed before aspartate. Videos of motile bacteria: 1) 2) 3) 4) 5) 6) 7) Free swimming bacteria Swimming in soft agar Tethered cells Latex bead tethered to flagellum Surface swarming behavior Swarm cells mixed with swim cells Aggregation / patterns formation Videos of motile bacteria: 1) Free swimming bacteria 2) 3) 4) 5) 6) 7) Swimming in soft agar Tethered cells Latex bead tethered to flagellum Surface swarming behavior Swarm cells mixed with swim cells Aggregation / patterns formation Watch for sudden changes of direction = tumbles Videos of motile bacteria: 1) Free swimming bacteria 2) Swimming in soft agar 3) 4) 5) 6) 7) Tethered cells Latex bead tethered to flagellum Surface swarming behavior Swarm cells mixed with swim cells Aggregation / patterns formation GFP labeled cells Cells are stuck most of the time but when the video is run at 5X they look almost like cells in aqueous environments. Videos of motile bacteria: 1) Free swimming bacteria 2) Swimming in soft agar 3) Tethered cells 4) 5) 6) 7) Latex bead tethered to flagellum Surface swarming behavior Swarm cells mixed with swim cells Aggregation / patterns formation wt - motor switches regularly cheY – motor does not switch cheZ – motor switched more frequently A cell is stuck to the coverslip by a sheared flagella. The motor still turns but since the flagella can’t the cell body rotates. Videos of motile bacteria: 1) Free swimming bacteria 2) Swimming in soft agar 3) Tethered cells 4) Latex bead tethered to flagellum 5) Surface swarming behavior 6) Swarm cells mixed with swim cells 7) Aggregation / patterns formation A cell is stuck to the coverslip and a latex bead is attached to a single flagella. The flagella rotation can be visualized by the bead. Videos of motile bacteria: 1) Free swimming bacteria 2) Swimming in soft agar 3) Tethered cells 4) Latex bead tethered to flagellum 5) Surface swarming behavior 6) Swarm cells mixed with swim cells 7) Aggregation / patterns formation Bacteria can move over a solid surface in a process call swarming. The movement is relatively slow compared to swimming and is coordinated. Videos of motile bacteria: 1) Free swimming bacteria 2) Swimming in soft agar 3) Tethered cells 4) Latex bead tethered to flagellum 5) Surface swarming behavior 6) Swarm cells mixed with swim cells 7) Aggregation / patterns formation Swarms cells are elongated relative to normal swimming cells. Videos of motile bacteria: 1) Free swimming bacteria 2) Swimming in soft agar 3) Tethered cells 4) Latex bead tethered to flagellum 5) Surface swarming behavior 6) Swarm cells mixed with swim cells 7) Aggregation / patterns formation Dilute cells placed under conditions where they release attractants will aggregate into large masses of cells (~30’ video ~2’). The Molecular Machinery of Chemotaxis INPUT Attractant concentration Signal Transduction OUTPUT Direction of rotation The Molecular Machinery of Chemotaxis INPUT Tsr Tar Tap Trg Attractants bind receptors at the cell surface changing their “state”. (methylated chemoreceptors MCPS). Signal Transduction OUTPUT Direction of rotation The Molecular Machinery of Chemotaxis INPUT Tsr Tar Tap Trg CheA (CheW) The MCPs regulate the activity of a histidine kinase - autophosphorylates on a histidine residue. P~ OUTPUT Direction of rotation The Molecular Machinery of Chemotaxis CheA transfers its phosphate to a signaling protein CheY to form CheY~P. INPUT Tsr Tar Tap Trg CheA (CheW) CheY P~ P~ OUTPUT Direction of rotation The Molecular Machinery of Chemotaxis CheY~P binds to the “switch” and causes the motor to reverse direction. The signal is turned off by CheZ which dephosphorylates CheY. INPUT Tsr Tar Tap Trg CheA (CheW) CheY CheZ P~ P~ OUTPUT Direction of rotation Excitatory Pathway At ‘steady state’, CheY~P levels in the cell are constant and there is some probability of the cell tumbling. Binding of attractant of the receptorkinase complex, results in decreased CheY~P levels and reduces the probability of tumbling and the bacteria will tend to continue in the same direction. MCP CheA (CheW) CheY~P CheZ Motor + attractant CheY inactive The Molecular Machinery of Chemotaxis Adaptation involves two proteins, CheR and CheB, that modify the receptor to counteract the effects of the attractant. INPUT Tsr Tar Tap Trg CheA (CheW) CheY CheZ CheR CheB P~ P~ OUTPUT Direction of rotation Adaptation Pathway CheR MCP CheA (CheW) Less active CheB~P MCP~CH3 CheA (CheW) More active Adaptation Pathway CheR MCP-(CH3)0 MCP-(CH3)1 MCP-(CH3)2 MCP-(CH3)3 MCP-(CH3)4 MCP-(CH3)0 MCP-(CH3)1 MCP-(CH3)2 MCP-(CH3)3 MCP-(CH3)4 +Attractant +Attractant +Attractant +Attractant +Attractant CheB~P In a receptor dimer there will 65 possible states (5 methylation states and two occupancy states per monomer). If receptors function in receptor clusters, essentially a continuum of states may exist. The conformational transition between T and R states of the MCP-CheACheW ternary complex probably involves an alteration in the positioning of methylated helices within a coiled coil structure. This transition is modulated by changes in the electrostatic potential between helices effected by the conversion of anionic glutamyl side chains to neutral methyl glutamyl groups and vice versa. Ligand binding between the sensory domain would act to perturb the T/R equilibrium by altering the relative positioning of monomers within the cytoplasm (see Fig. 6). This interplay between methylation and stimulation could operate to control the relative positioning of signaling domains and their associated CheA subunits so as to regulate the transphosphorylation activity of CheA, which through CheY controls the swimming behavior of the bacterial cell. Some Issues in Chemotaxis: • Sensing of Change in Concentration not absolute concentration i.e. temporal sensing • Exact Adaptation • Sensitivity and Amplification • Signal Integration from different Attractants/Repellents The range of concentration of attractants that will cause a chemotactic response is about 5 orders of magnitude (nM mM) References on Modeling Chemotaxis Barkai, N. & Leibler, S. (1997) Nature (London) 387: 913–917. Spiro, P. A., Parkinson, J. S. & Othmer, H. G. (1997) Proc. Natl. Acad. Sci. US 94: 7263–7268. Tau-Mu Yi, Yun Huang , Melvin I. Simon, and John Doyle (2000) Proc. Natl. Acad. Sci. USA 97: 4649–4653.* Bray, D., Levin, M. D. & Morton-Firth, C. J. (1998) Nature (London) 393: 85–88. * * - these models have incorporated the Barkai model. Robustness in simple biochemical networks N. Barkai & S. Leibler Departments of Physics and Molecular Biology, Princeton University, Princeton, New Jersey 08544, USA Simplified model of the chemotaxis system. Mechanism for robust adaptation E is transformed to a modified form, Em, by the enzyme R; enzyme B catalyses the reverse modification reaction. Em is active with a probability of am(l), which depends on the input level l. Robust adaptation is achieved when R works at saturation and B acts only on the active form of Em. Note that the rate of reverse modification is determined by the system’s output and does not depend directly on the concentration of Em (vertical bar at the end of the arrow). Some parameters used to characterize the network. Tumble frequency Steady-State Tumble Frequency Adaptation Time Adaptation precision Chemotactic response and adaptation in the Model. The system activity, A, of a model system which was subject to a series of step-like changes in the attractant concentration, is plotted as a function of time. Attractant was repeatedly added to the system and removed after 20 min, with successive concentration steps of l of 1, 3, 5 and 7 mM. Note the asymmetry to addition compared with removal of ligand, both in the response magnitude and the adaptation time. How robust is the model with respect to variation in parameters? Adaptation precision Adaptation Time Adaptation precision (i.e. exact adaptation) is Robust Adaptation time is very sensitive to parameters Testing the predictions of the Barkai model Robustness in bacterial chemotaxis. U. Alon, M. G. Surette, N. Barkai & S. Leibler • The concentration of che proteins were altered as a simple method to vary network parameters. • The behavior of the cells were measured (adaptation precision, adaptation time and steady-state tumble frequency). • In each case the predictions of the model we observed. Data for CheR As predicted by the model the adaptation precision was robust while adaptation time and steady-state tumble frequency were very sensitive to conditions. Regulation of flagella gene expression: A three tiered transcriptional hierarchy Positive transcriptional regulators Alternative sigma factors Ant-sigma factors Temporal regulation The Flagellar Transcription Hierarchy 1. The Master Regulon 2. The FlhCD Regulon CRP,H-NS,OmpR other? Chemotaxis proteins Motor proteins FlhCD inside outside FlgM FliA Basal Body and Hook other? 3. The FliA Regulon Filament The flhDC promoter integrates inputs from multiple environmental signals flhDC ? CRP - catabolite repression, carbohydrate metabolism OmpR - osmolarity IHF - growth state of cell? HdfR - ? FliA Regulation by FlgM FlhDC expression leads to activation of Level 2 genes including the alternative sigma factor FliA and an anti sigma factor FlgM FlgM accumulates in the cell and binds to FliA blocking its activity (i.e. interaction with RNA polymerase) preventing Level 3 gene expression. inside outside Level 3 Genes FliA Regulation by FlgM Other level 2 genes required for Basal body and hook assembly are made and begin to assemble in the membrane. Level 3 Genes inside outside Basal Body and Hook Assembly FliA Regulation by FlgM The Basal body and hook assembly are completed. Level 3 Genes inside outside Completed Basal Body and Hook FliA Regulation by FlgM The Basal body and hook assembly are completed. FlgM is exported through the Basal Body and Hook Assembly Level 3 Genes inside outside Completed Basal Body and Hook FliA Regulation by FlgM Level 3 gene expression is initiated. FlgM is exported through the Basal Body and Hook Assembly. FliA can interact with RNA polymerase and activate Level 3 gene expression. Level 3 Genes inside outside Completed Basal Body and Hook FliA Regulation by FlgM Level 3 gene products are added to the motility machinery including the flagella filament, motor proteins and chemotaxis signal transduction system. inside outside Filament Using reporter genes to measure gene expression RNA polymerase Regulator Organization of operon on chromosome. flhD flhDC promoter flhC Using reporter genes to measure gene expression RNA polymerase Regulator Organization of operon on chromosome. flhD flhC flhDC promoter Clone a copy of the promoter into a reporter plasmid. Reporter gene Using reporter genes to measure gene expression RNA polymerase Regulator flhD flhC Both the flhDC genes and the reporter plasmid are regulated in the same way and thus the level of the reporter indicates the activity of the promoter. Reporter gene Note that the strain still has a normal copy of the genes. Gene Expression in Populations Gene Expression in Single Cells Multi-well plate reader Video microscopy - sensitive, fast reading - high-throughput screening - liquid cultures - colonies - mixed cultures - “individuality” - cell cycle regulation - epigenetic phenomenon Automation: Both approaches are amenable to high throughput robotics Fluorescence of flagella reporter strains as a function of time Fluorescence relative to max 0.6 0.1 0.01 0 600 Time [min] The order of flagellar gene expression is the order of assembly Early Cluster 1 Class 1 flhDC Cluster 2 Late Class 2 fliL Class 2 fliE Class 2 fliF Class 2 flgA Class 2 flgB Class 2 flhB Class 2 fliA Class 3 fliD Class 3 flgK Class 3 fliC Class 3 meche Cluster 3Class 3 mocha Class 3 flgM Master regulator Activator of class 3 Simple Mechanism for Temporal Expression Within an Regulon [protein] Induction of positive regulator Time Promoters with decreasing affinity for regulator Simple Mechanism for Temporal Expression Within an Regulon [protein] Using Expression Data to Define and Describe Regulatory Networks With the flagella regulon, current algorithms can distinguish Level 2 and Level 3 genes based on subtleties in expression patterns not readily distinguished by visual inspection. Using our methods for expression profiling (sensitive, good time resolution) we have been able to demonstrate more subtle regulation than previously described. The Challenge: Can this type of experiment and analysis be used to describe the details of the flagella regulon? (our ‘model’ network) Can this be applied on a genomic scale? Synchronization of the population occurs only under some growth conditions 0 600 0 600 Time [min] Condition A Time [min] Condition B (No pre-existing flagella) (Pre-existing flagella) 1:600 dilution 1:60 dilution flhDC activation Level 2 genes Level 2 & 3 genes Level 3 genes Variability in 22 E. coli flhDC Promoters 500000 400000 Relative Promoter Activity (max) 300000 200000 100000 0 *** 1000000 100000 10000 1000 100 *** The Promoter for flhDC varies significantly between E. coli Isolates • In several randomly cloned E. coli flhDC promoters, there is a large distribution in promoter strength • Quantitative differences in promoter strength can not be inferred from promoter sequence nor from swim rates on soft agar plates. • The same promoter behaves differently in different strain backgrounds which implies variability in regulators acting on the promoter (CRP,OmpR etc.) • Correct temporal patterning of gene expression and assembly of flagella occurs despite significant variation in the level of gene expression between strains. Where is the source of the ‘robustness’ in this genetic network?