* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Week 5
Survey
Document related concepts
Transcript
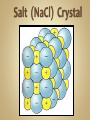
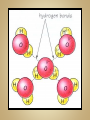
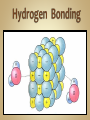
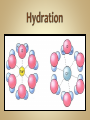
Solutions & The Ocean Professor Bob Kaplan University Department of Science Most of the things you see and feel every day are chemical compounds. Compounds consist of a combination of atoms of two or more elements. Although there are 115 elements known today, there are millions of compounds due to the many ways atoms combine, with thousands more discovered each year. Electrons are shared equally by the atoms. Most hydrogen compounds such as water H2O are covalently bonded. 70% of earth’s surface is covered by water. The human body is about 60-65% water. Organic (C, H, O) compounds include: Carbohydrates, Fats, Proteins, DNA Covalent bonding Chemical bond: A force that holds groups of atoms together & makes them function as a unit. Bond energy is the energy required to break the bond. Ionic bonds: Strong forces of attraction between positively charged cations and negatively charged anions which hold the ions together in a crystalline lattice Formed when an atom that readily loses electrons reacts with an atom that has a high electron affinity. Metal reacts w/ Non-metal Electrons are transferred from one atom to another to form positive cations and negative anions. Cl will gain one electron to be like Ar Cl combines with Group I element ( Na ) Cl atom Cl- anion Na loses one electron to be like Ne Na combines with Group VII element ( Cl ) Na atom Na+ cation Bond polarity is a simple result of the fact that: Electrons are not shared equally. In a polar covalent bond, the atom with the stronger affinity for electrons may be shown with a partial negative charge. The atom with the lower affinity for electrons is farther from the electron pair and is shown with a partial positive charge. Illustration of the hydrogen fluoride (HF) molecule. The dipole is symbolized by a cross (+ end ) . Note the arrowhead pointing in the direction of the negative end. Note the larger size of the molecule on the negative end, as the unequally shared electrons are spending more time there. Polar molecules of any substance have attractions between the positive end of one molecule and the negative end of another molecule. When the polar molecule has hydrogen at one end and fluorine, oxygen or nitrogen at the other end, the attractions are strong enough to qualify as type of chemical bonding called: Hydrogen bonding A hydrogen bond is an attraction between the oxygen atom of one water molecule and a hydrogen atom in another. Hydrogen bonds are much weaker than covalent or ionic bonds. Hydrogen bonding plays an important role in the properties of water and biochemical compounds such as amino acids, proteins & DNA. A solution is a homogeneous mixture in which one substance called the solute is dispersed uniformly in another substance called the solvent. Water acts as the solvent (major component) for the solute (minor component) or the substance that is dissolved or dispersed in the solvent in aqueous solutions. The air we breathe is a solution of oxygen and nitrogen gases. When we make solutions of coffee or tea, we use hot water to dissolve substances from coffee beans or tea leaves. In a hospital, the antiseptic tincture of iodine is a solution of iodine dissolved in alcohol. The ocean is an aqueous solution of many salts such as NaCl dissolved in water. Dilute solutions contain very little solute. Concentrated solutions contain a larger quantity of solute. Body fluids contain solute ions such as K+ , Na+ , Cl- , H+ , HC03Solvent: Blood plasma Significant changes in their concentrations can be a sign of illness or injury. Solutions, solutes and solvents may be combinations of solids, liquids or gases. The solution that forms has the same physical state as the solvent. Carbonated beverages are formed by dissolving CO2 gas in water. Stainless steel is a solid solution of carbon atoms in a crystalline matrix of iron atoms. Solutions dissolve a limited amount of solute. A solution that has not reached the limit of solute that will dissolve in it is said to be unsaturated. When a solution contains as much solid as will dissolve at that temperature, it said to be saturated. NaCl will form a solution with water because the Na+ and Cl- ions in the salt are attracted to the positive and negative parts of water molecules. In the hydration of NaCl : Cl- ions are attracted to partially ( + ) H atoms, Na+ ions are attracted to partially ( - ) O atoms. This pulls charged ions from the crystalline solid, as they become surrounded by water molecules. When ionic compounds dissolve, the resulting solution contains separated ions. The conduction of electricity provides evidence for these ions in solution. Charged ions in solution act as mobile charge carriers (like electrons in metals). Sodium chloride and water together form a strong electrolyte aqueous solution. Solutes that dissolve in water as covalent molecules do not conduct electricity . Table sugar dissolves as a polar molecule, but does not dissociate into charged ions. Why is ethanol so soluble in water? The ethanol molecule contains a polar O-H group, which makes it compatible with water. Covalently bonded substances such as elemental gases, long chain hydrocarbon fuels, oil or grease do not dissolve in water or other polar solvents. Non-polar solutes require non-polar solvents. ‘Like dissolves Like’ In a covalently bonded compound such as petroleum oil, the bonding electrons are shared equally. Covalent bonds are essentially non-polar. The resulting molecule with its non-polar bonds cannot form attractions to polar water molecules. This prevents it from being soluble in water. If you have come here directly from the SC155 Seminar session, please return to the KU course platform now to continue with the live session of discussion, questions and answers See you all there ! 31