* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Introduction to Toxicology
Psychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Blood doping wikipedia , lookup
Prescription costs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Neuropharmacology wikipedia , lookup
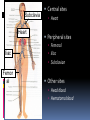
بنام خداوند بخشنده مهربان FORENSIC TOXICOLOGY دكرت فرزاد قشالقي متخصص طب قانوين و مسموميتها ِ دانشار دانشکده پزشکی E. mail:[email protected] Introduction to Toxicology Toxicology What is toxicology? The study of the effects of poisons. Poisonous substances are produced by plants, animals, or bacteria. Phytotoxins Zootoxins Bacteriotoxins Toxicant - the specific poisonous chemical. Xenobiotic - man-made substance and/or produced by but not normally found in the body. Introduction Toxicology is arguably the oldest scientific discipline, as the earliest humans had to recognize which plants were safe to eat. Most exposure of humans to chemicals is via naturally occurring compounds consumed from food plants. Humans are exposed to chemicals both inadvertently and deliberately. History 2700 B.C. - Chinese journals: plant and fish poisons 1900-1200 B.C. - Egyptian documents that had directions for collection, preparation, and administration of more than 800 medicinal and poisonous recipes. 800 B.C. - India - Hindu medicine includes notes on poisons and antidotes. 50-100 A.D. - Greek physicians classified over 600 plant, animal, and mineral poisons. History 50- 400 A.D. - Romans used poisons for executions and assassinations. The philosopher, Socrates, was executed using hemlock for teaching radical ideas to youths. Avicenna (A.D. 980-1036) Islamic authority On poisons and antidotes. 1200 A.D. - Spanish rabbi Maimonides writes first-aid book for poisonings, Poisons and Their Antidotes History Swiss physician Paracelsus (14931541) credited with being “the father of modern toxicology.” “All substances are poisons: there is none which is not a poison. The right dose differentiates a poison from a remedy.” The Dose Makes the Poison An apparently nontoxic chemical can be toxic at high doses. (Too much of a good thing can be bad). Highly toxic chemicals can be life saving when given in appropriate doses. (Poisons are not harmful at a sufficiently low dose). History Italian physician Ramazzini (1713) published “De Morbis Artificum” (Diseases of Workers) describing "asthma" in bakers, miners, farmers, gilders, tinsmiths, glass-workers, tanners, millers, grain-sifters, stonecutters, ragmen, runners, riders, porters, and professors. Ramazzini outlined health hazards of the dusts, fumes, or gases that such workers inhaled. The bakers and horse riders described by Ramazzini would today probably be diagnosed as suffering from allergen-induced asthma. The lung diseases suffered by most of the other workers would now be classified as "pneumoconiosis," a group of dust-related chronic diseases. History Spanish physician Orfila (1815) established toxicology as a distinct scientific discipline. FORENSIC TOXICOLOGY Forensic Toxicology Toxicology is defined as the study of the adverse effects of chemicals on living organisms. Forensic toxicology is defined as the application of toxicology for the purposes of the law. History Ancient Egyptians and Grecians reported poisonings due to herbs, plants and food. Opium, arsenic and hydrocyanic acid were used throughout Europe during the middle ages. History Philippus Theophrastus Aureolus Bombastus von Hohenheim (or Paracelsus) observed that any substance could be a poison, depending on its dose “ What is there that is not poison? All things are poison and nothing without poison. Solely the dose determines that a thing is not a poison” Paracelsus (1493-1541), more properly Phillippus Theophrastus Aureolus Bombastus von Hohenheim, was born in Einsiedeln, Switzerland in 1493, one year after Columbus' first voyage to the New World. He was a contemporary of Copernicus, Martin Luther, Leonardo da Vinci and a host of other figures we associate with the shattering of medieval thought and the birth of the modern world. History In 1814, M.J.B. Orfila, the chairman of the legal medicine department at the Sorbonne in France, published a book entitled Traite des poisons ou Toxicologie Generale. This was the first attempt to systematically study and classify poisons. Six classes of poisons were established, based mainly on their toxic effects. He also isolated arsenic from a variety of postmortem specimens and was the first to articulate the fact that poisons must be absorbed, or enter the blood, to manifest their toxic effects. History In 1851, Stas developed the first effective method for extracting alkaloids from biological specimens. This was modified several years later by Otto, which enabled the isolation of purer alkaloid substances. This became known as the StasOtto method and remains the basis for drug extraction to this day. In the U.S., forensic toxicology did not develop until the early 20th century. In the U.S., forensic toxicology did not develop until the early 20th century, with the change of the coroner system in New York to a medical examiner system. The first forensic toxicologist was Dr. Alexander Gettler, who directed the laboratory for 41 years and trained the first generation of forensic toxicologists in the country. Dr. Alexander Gettler is considered this country’s first forensic toxicologist. Forensic Toxicology Postmortem forensic toxicology. Human performance toxicology. Forensic drug testing. Outline Definitions and purpose of postmortem tox Samples of forensic interest Handling and storage of samples Pitfalls in postmortem toxicology Interpretation of results Postmortem Forensic Toxicology Death Investigations(Medicolegal sys.) Coroner Medical Examiner Continental A coroner may be elected by the people or appointed by a governmental authority. A medical examiner is appointed, usually by the health department. A coroner is not required to have any particular training or experience in medicine. Conversely, a medical examiner must be a physician, usually a pathologist, with specific training in forensic medicine Postmortem Forensic Toxicology Qualitative and quantitative analysis of drugs or poisons in biological specimens collected at autopsy Interpretation of findings in terms of: Physiological effect at time of death Behavioural effect at time of death How might one define postmortem forensic toxicology? The purpose of postmortem forensic toxicology is to perform qualitative and quantitative analysis for drugs and their metabolites, and poisons such as metals, carbon monoxide, and volatile substances in human fluids and tissues collected after death. Quantitative vs. Qualitative Qualitative analysis – determines the presence or absence of a drug or poison in a submitted sample Quantitative analysis – determines the amount of drug or poison that is present in the submitted sample Postmortem Forensic Toxicology Types of cases: Suspected drug intoxication cases Fire deaths Homicides Driver and pilot fatalities Therapeutic drug monitoring Sudden infant death (SIDS) These are the types of cases that typically fall into the realm of postmortem forensic toxicology. 1. Unexplained deaths, with no apparent cause (often suspected to be drug intoxication cases) as well as those that are strongly suspected of being drug intoxication cases. 2. Fire deaths – measurment of toxic gases such as carbon monoxide and cyanide which may be inhaled during a fire. Furthermore, drugs may be implicated as having incapacitated a victim, thereby preventing their escape from a fire. 3. Homicides – homicidal poisonings themselves, are rare – but many homicide cases are related to drug use and drug abuse. 4. Driver and pilot fatalities – where drug impairment may help determine the cause of a crash. Other traumatic causes of death will also require postmortem toxicology analysis (such as drownings, falls) 5. Therapeutic drug monitoring – for example, determining whether an individual with a seizure disorder has been compliant in their medication use. 6. SIDS – by definition, SIDS is a diagnosis of exclusion. Therefore, toxicology must be comprehensive in these case to rule out any other cause of death. Issues in Specimen Collection Selection Multiple, varied sites of collection Collection Appropriate method of collection Adequate volumes for analysis Storage and handling Important to ensure analytical results are accurate and interpretations are sound SAMPLES OF FORENSIC INTEREST Typical autopsy specimens Blood Urine Stomach contents Bile Liver Hair Vitreous humor Blood Antemortem ideal blood sample Postmortem blood is not truly “blood” Anatomical site of collection at autopsy should be noted Subclavia n Heart Central sites Heart Peripheral sites Femoral Iliac Iliac Subclavian Femor al Other sites Head blood Hematoma blood Ideally, blood should be collected from both a central site, such as from the chambers of the heart – in addition to one or more samples being collected from a peripheral site. The most common peripheral sites of blood collection are femoral, iliac, and subclavian (emphasis on femoral). “Head blood” – rarely seen in adults, but is not an uncommon site of selection in babies, especially since in infants it is difficult to obtain blood from the peripheral sites and blood volume of the heart is small. Hematoma blood – Hematoma Extravascular blood clot Protected from metabolism Analysis will indicate what drugs were present in the blood at the time of formation Analysis of hematoma blood has has proven especially useful with alcohol… Hematoma case example A 62year old man was found dead at the bottom of a staircase. Death was due to physical injuries. Question as to alcohol use prior to fall down stairs No urine available at autopsy Alcohol not detected in femoral blood Alcohol in hematoma blood 150 mg/100 mL The deceased had been drinking prior to receiving the head trauma. The deceased had survived for several hours after the injury. Hematoma Caution: There may be a delay between the incident which resulted in hematoma and the actual formation of the hematoma Therefore, this alcohol concentration does not necessarily indicate the BAC at the time of the fall down the stairs. Urine Produced by the kidneys Blood filtered by the kidneys Stored in the bladder until voided Qualitative - the presence of a drug in the urine of an individual indicates that some time prior to death the drug or poison was present in the blood of the individual It simply means that some time prior to death the drug or poison was present in the blood. urine analysis – in isolation of blood analysis is of limited value. Stomach contents Visual examination may reveal tablets Drugs that have been orally ingested may be detected in stomach contents Caution: drugs administered by other routes may also diffuse into stomach contents from the blood(pH gradient) Generally qualitative: Stomach contents are not homogeneous Only a portion of stomach contents collected (unmixed?) Useful for directing further analysis Case Example A 62 year old woman is found dead in bed Numerous medications in her home: Amitriptyline, Oxycodone, Morphine, Paroxetine, Diphenhydramine, Pseudoephedrine, Phenobarbital, Codeine, Temazepam, Diazepam Only 3 mL of blood collected at autopsy Qualitative analysis of stomach contents: Amitriptyline: detected Nortriptyline: detected Quantitation can now be performed in blood Liver Drug metabolism occurs in the liver Both parent compounds and metabolites may be present in higher concentrations in the liver than in the blood ease of detection Limitation is that drugs are not uniformly distributed throughout the liver confounds interpretation Bile Digestive secretion Continuously produced by the liver Stored in the gallbladder Qualitative - the presence of a drug in the bile of an individual indicates that sometime prior to death, the individual was exposed to the drug Vitreous humor Fluid that occupies the space between the lens and the retina of the eye. Sequestered from putrefaction, charring and trauma, microorganisms. Useful in cases where decomposition is advanced, body is exhumed or in fire deaths Limitation is blood:vitreous ratio may not be known Hair Recent specimen of interest Metabolism does not occur in hair Can provide a historical record(generally 1 cm/month) of drug or poison exposure Pros and cons of hair analysis still being uncovered racial variability? Case Example 70 year old woman, previously in good health Nausea, vomiting, diarrhea, rash, fever Weakness in hands and feet Guillian Barre? Hospitalized with hypotension, seizures Misplaced laboratory result Arsenic! Sequential hair analysis for arsenic showed chronic arsenic poisoning over 8 month period Non-biological submissions Used to direct analysis of biologicals May indicate the nature of substances that may have been ingested, inhaled or injected Examples: Containers found at the scene Syringes Unidentified tablets or liquids Autopsy specimens of limited value Pleural fluid Chest cavity blood Gutter blood Samples taken after embalming Samples taken after transfusion in hospital • “Spleen squeezings” • “Esophageal scrapings” Chest Cavity Fluid Not readily definable Most likely to be collected if: Traumatic injury to the chest Advanced decomposition A “contaminated” blood sample, chest cavity fluid may contain fluids from stomach, heart, lungs etc. Samples taken after embalming Methanol is a typical component of embalming fluid Most drugs are soluble in methanol Embalming process will essentially “wash” the vasculature and tissues Qualitative analysis can be performed on body tissues Case Example A 72 year old woman, given meperidine to control pain following surgery, later died in hospital. The woman was in poor health and it is possible that death was due to natural causes. However, coroner requests toxicology to rule out inappropriate meperidine levels. BUT: Body had been embalmed Liver and spleen submitted STORAGE AND HANDLING Proper specimen handling Identification of samples Continuity Contents Specimens delivered to lab without delay Specimens should be analyzed as soon as possible Storage areas should be secure Storage and Handling Not possible to analyze specimens immediately Sample should be in well-sealed container Sample containers must be sterile Use of preservatives and anti-coagulants Refrigeration vs. Freezing Both inhibit bacterial action; esp. freezing Freezing results in prep time Freeze-melt cycle may promote breakdown Storage of Samples Preservative Sodium fluoride Anti-coagulants are not really necessary in postmortem blood samples since the blood is hemolyzed! Sodium citrate Potassium oxalate EDTA Heparin Not imperative for postmortem blood samples Determining analyses Case history Medical history Autopsy findings Symptomatology Experience of the toxicologist Amount of specimen available Nature of specimens available Policies of the organization PITFALLS IN POSTMORTEM FORENSIC TOXICOLOGY Decomposition first and most visible problem facing postmortem forensic toxicology 1.Autolysis The breakdown of cellular material by enzymes 2.Putrefaction A septic/infectious process The destruction of soft tissues by the action of bacteria and enzymes Traumatic deaths may demonstrate putrefaction Decomposition Fewer samples available for collection Quality of samples is diminished Putrefaction produces alcohols Ethanol Isopropanol Acetaldehyde n-propanol Postmortem redistribution A phenomenon whereby increased concentrations of some drugs are observed in postmortem samples and/or site dependent differences in drug concentrations may be observed Typically central blood samples are more prone to postmortem changes (will have greater drug concentrations than peripheral blood samples) Possible mechanisms of postmortem redistribution 1.Diffusion from specific tissue sites of higher concentration (e.g. liver, myocardium, lung) to central vessels in close proximity 2.Diffusion of unabsorbed drug in the stomach to the heart and inferior vena cava 3.Diffusion of drugs from the trachea, associated with agonal aspiration of vomitus Case Example • 37 year old man found dead in his home • Cause of death identified at autopsy as asphyxia due to choking; white pasty material lodged in throat Heart blood Morphine: 20 000 ng/mL Amitriptyline: 0.36 mg/dL Femoral blood Morphine: 442 ng/mL Amitriptyline: 0.01 mg/dL • Examination of esophageal and tracheal contents revealed presence of both morphine and amitriptyline Susceptible Drugs Drugs most commonly associated with postmortem redistribution: are chemically basic 2. have large volumes of distribution 1. Volume of distribution Review from last lecture: Volume of distribution is the amount of drug in the whole body (compared to the amount of drug in the blood) If a drug has a large volume of distribution, it is stored in other fluids and tissues in the body Susceptible Drugs Tricyclic Narcotic antidepressants Analgesics Codeine Oxycodone Propoxyphene Amitriptyline Nortriptyline Imipramine Desipramine Antihistamines Diphenhydramine Doxepin Digoxin Postmortem redistribution Coping with the problem of postmortem redistribution: Analysis of both central blood and peripheral blood in cases where postmortem redistribution may be a factor Compilation of tables to determine average and range of postmortem redistribution factors for drugs Incomplete Distribution Site dependent differences in drug levels due to differential distribution of drugs at death Has been noted in rapid iv drug deaths Example: Intravenous injection of morphine between the toes Fatal amount of drug reaches the brain Full distribution of the morphine throughout the body has not occurred Femoral concentration > Heart concentration Drug Stability Knowledge of a drug’s stability is necessary to facilitate interpretation of concentrations Breakdown of drugs may occur after death and during storage via non-enzymatic mechanisms Cocaine Benzoylecgonine (Hydrolysis) LSD degradation due to light sensitivity Others ? Evaporation of volatiles Ethanol Carbon monoxide Cyanide Toluene Other alcohols Example: Carbon Monoxide Effects of storage conditions on stability of CO No significant change in % CO saturation in capped samples stored at room temperature or 4oC Significant losses in % CO saturation in uncapped samples stored at room temperature and at 4oC Mechanism for loss diffusion INTERPRETATION Interpretation Therapeutic, toxic or fatal? How do you know? Compare measured blood concentrations with concentrations reported in the literature: Clinical pharmacology studies Incidental drug findings Plasma blood Consider case history: Symptoms observed by witnesses? Tolerance of the individual to the drug Blood:plasma ratios Knowledge of the blood:plasma ratio can be very important when applying information from clinical studies to postmortem forensic tox Cocaine, blood:plasma ratio is 1.0 Phenytoin, blood:plasma ratio is 0.4 Ketamine, blood:plasma ratio is 1.7 Hydroxychloroquine, blood:plasma ratio is 7.2 Example: THC Six healthy male volunteers recruited for a study of the pharmacokinetics of THC in humans Smoked a “high-dose” THC cigarette 15 minutes after cessation of smoking, plasma THC concentrations averaged 94.8 ng/mL The plasma:blood ratio for THC is 1.8 Plasma contains 1.8x as much THC as whole blood The results of this study correspond to a blood THC concentration averaging 53 ng/mL Importance of History: Tolerance Drug concentrations in non-drug related deaths may overlap with reported drug concentrations in fatal drug intoxications Methadone example: Naïve users - deaths due to methadone are associated with blood levels > 0.02 mg/100 mL Patients on methadone maintenance – peak blood concentrations may range up to 0.09 mg/100 mL Interpretation Acute vs. Chronic Ingestion: Can you tell? Parent:metabolite drug concentration ratio may be of assistance in differentiating between acute and chronic ingestion of a drug Example: Amitriptyline Case 1 Amitriptyline: 0.4 mg% Nortriptyline: 0.02 mg% Parent >> Metabolite Suggestive of acute overdose and rapid death Case 2 Amitriptyline: 0.04 mg% Nortriptyline: 0.08 mg% Parent < Metabolite Slow death and/or chronic administration Interpretation Metabolites are produced when drugs are biotransformed (converted) into other chemicals, more easily excreted from the body Metabolite drug concentrations may be the more useful measure of exposure or toxicity Metabolites: Exposure The parent compound may be a prodrug or may have a shorter t1/2 than the metabolite: Clorazepate nordiazepam Flurazepam N-desalkylflurazepam Heroin morphine Metabolites: Toxicity The metabolite may have toxicity over the parent compound: Acetaminophen N-Acetylbenzoquinoneimine Meperidine normeperidine Methanol formic acid Ethylene glycol oxalic acid calcium oxalate Human Performance Toxicology Human performance toxicology is also referred to as behavioral toxicology. It is the study of human performance under the influence of drugs. This branch of forensic toxicology is concerned with the relationship between the presence of a drug and associated behavioral changes. It is generally accepted that there is a dose- effect relationship between drugs that elicit behavioral changes and those changes; elucidation and quantification of such a relationship is a significant role of the behavioral toxicologist. Human Performance Toxicology Ethanol and driving History Behavioral effects Specimens Human Performance Toxicology Drug Recognition Evaluation (continued) Toxicology Type of Testing Specimens Human Performance Toxicology Drug Recognition Evaluation Drug Class Effects Central Nervous System Depressants Central Nervous System Stimulants Hallucinogens Phencyclidine Narcotic Analgesics Inhalants Cannabis Forensic Drug Testing Uses in the workplace: Pre-employment screening Post-accident testing Return to Work testing “For Cause” testing Random testing Man is the maker of his own happiness..