* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Medivir
Clinical trial wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Prescription costs wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
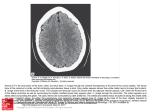
Discovery and development of HIV-protease inhibitors wikipedia , lookup
Medivir AB Acumen BioFin 4th Annual Global Healthcare Conference Lars Adlersson, CEO & President Basic facts • Listed since 1996 (OME: MVIRB SS) 18 month performance • MCap: ~ USD 180m • Major shareholders: Staffan Rasjö Founders Handelsbanken Den Norske Bank Skandia • Cash position: SEK 370m (end-Q1) • Revenues: SEK 129m (FY 2006) • Burn rate: SEK 155m (FY 2006) 2 The strategic journey Nordic marketing rights for all projects and possible product from J&J Successful outlicensing of 6 polymerase projects. Strong partnerships with deal value in excess of 400 MUSD plus royalties 3 Pipelines and partners 4 Key projects LABIAL HERPES HEPATITIS C OSTEOPOROSIS Lipsovir®, Phase III data late 2007. Market approval by end of 2008. Collaboration with Tibotec / Johnson & Johnson. Phase I trials ongoing. MIV-701, Phase I trials ongoing. Further indications such as OA, RA and bone metastases explored. 5 Labial herpes (Lipsovir®) • First drug to treat and prevent cold sores • Low-risk — based on safe, well-documented and already marketed compounds • Goal: Marketing permission from regulatory authorities by 2008 6 Herpes virus Immune defense 7 Herpes virus Current drugs Immune defense 8 Herpes virus Lipsovir® Immune defense 9 ® Lipsovir : first to show prevention Prevention of cold sores Reduction in lesion healing time Acyclovir No 10 - 12% Penciclovir No 13 - 15% Docosanol (Abreva) No (15%) Acyclovir No 9 - 13% Valaciclovir No 10 – 11% Yes, 29% 11% (19%*) Treatment Topical vs placebo Oral vs placebo Lipsovir® vs placebo * Episode duration 10 A billion dollar market Annual value growth: 9% Rx (USD 450m) Annual value growth: 12% Palliative (USD 400m) OTC (USD 220m) 11 Development process 60% treated July 06 Jan 07 Start Phase III Mar 07 Phase III data H2 07 H1 08 75% treated Approval end-08 Filing 2009 Genital Herpes? Marketing Partner(s) 12 Three of four sufferers express ® an interest in Lipsovir Definitely would buy Probably would buy Might or might not buy Probably would not buy Definitely would not buy Primary market research 403 (UK) 411 (US) respondents 13 Physician prescribing habits post® launch of Lipsovir • Physicians are willing to prescribe a new cold sore remedy • When asked what they would prescribe on the next 10 occasions, Lipsovir claims a 45% share of prescriptions, making it the leading product and drawing users from all 3 principal Rx brands (Zovirax, Valtrex, Denavir) Primary market research Base: 225 physicians who have prescribed cold sore remedies 10+ times in the last 12 months 14 Hepatitis C – HCV PI Market • 170-200 million infected • Over 50% non-responders to current treatments • Estimated market value in 2010: 7.8 billion USD Key enzyme for virus replication Process • Partnership with Tibotec / Johnson & Johnson since November 2004 • Candidate drug selected 2005 • CTA submitted December 2006 • Phase I trials started February 2007 Patents • Extensive and non-limiting IP published July 2005 Enzyme inhibiting compound 15 Medivir/Tibotec HCV PI Series • Several series of highly promising NS3/4A inhibitors have been developed Data on Compound “A” (an example from the lead series) 1 R 2 R L H N O M O O <1nM Replicon activity on genotype 1b [IC50, nM] <10nM 3 R ( )n • Enzymatic activity on genotype 1b (Ki, nM) Further optimization of these inhibitors in collaboration with Tibotec Pharmaceuticals Ltd, J&J, has resulted in the selection of a Clinical Candidate In vivo PK, Oral dose: 10 mg/kg Cmax (µM) T 1/2 (h) F (%) 0.95 5.4 20 From presentations at the 1st International Workshop on Hepatitis C Resistance and New Compounds, Boston 25-26 October 2006 and the 3rd Anglo-Swedish Medicinal Chemistry Meeting, 11-14 March 2007, Åre, Sweden 16 Bone disorders (MIV-701) • MIV-701 selectively inhibits the bone and cartilage degrading enzyme cathepsin K • Osteoporosis, osteoarthritis and bone metastases Bone surface • Target profile: • Improved bone quality (c/f bisphosphonates) • Bone growth capability Cath K • Once-daily oral dosing • Strong Back-up program in place with pre-CD’s selected Osteoclast 17 Bone disorders (MIV-701) Market • Approx 100 million patients in major growing markets (osteoporosis only) • Global osteoporosis market 11 billion USD by 2008 • Strong interest in cathepsin K inhibition from major pharma companies Process • Clinical phase Ia trials commenced March 2007 • Phase Ib trials planned for late 2007 Patent/generic competition • Patent applications being processed • Expected patent protection until 2025 Partner strategy • Establish industrial partnership after completion of phase Ib (2008) 18 “The Protease Discovery Engine”: A reliable repeat innovator HIV – PI –Collaboration project with Tibotec / Johnson & Johnson MMP- COPD –Collaboration with Hengrui –Extensive IP, excellent results in pre-clinical disease model –Next step: selection of Candidate Drug Renin - Hypertension –IP compiled for three distinct and potent inhibitor series –Next step: studies in a pre-clinical efficacy model Cathepsin S – RA, MS and pain –Potent and selective inhibitors –Efficacious in preclinical disease models –Fine-tuning of PK properties Several early protease programs, e.g. Alzheimer's (BACE inhibitors) and novel cholesterol lowering MOA Large inhibitor libraries and proprietary technologies –facilitate CD generation against any new protease target 19 The journey ahead LIPSOVIR HEPATITIS C MIV-701 HIV FRANCHISE • Phase III data, autumn 2007 • Partnership agreement(s) • Market registration, end-2008 • Phase I data during 2007 • Possibility to receive “approved drug” from Johnson & Johnson • Phase I data during 2007 • Partnership post phase I • MIV-170: Entry into phase I by late 2007 • MIV-606 start of phase IIb trials • New clinical trials and new data in other outlicensed projects 20 Next step in company transformation A profitable pharmaceutical company with its own research and sales