* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
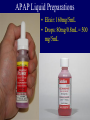
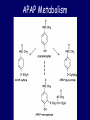
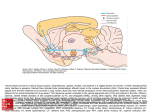
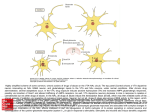
ACETAMINOPHEN Steven A. Seifert, MD, FACMT, FAACT Professor, Medical Toxicology University of New Mexico School of Medicine Medical Director New Mexico Poison & Drug Information Center [email protected] Karen, PharmD Sara, PharmD, CSPI Rose, PharmD, CSPI Robert, PharmD, CSPI Stevie, PharmD, CSPI Holly, PharmD, CSPI Ina Bawaya, Educator Drug Info. Jess Benson, Steven Seifert, MD PharmD, DABAT, Medical Director Managing Director Lee, PharmD, CSPI Jennifer, PharmD, CSPI LaDonna PharmD, CSPI Damon, PharmD, CSPI Gordon, PharmD, CSPI Lauri Ivy, Admin Asst. Common Things Are Common • Analgesics (2008 NPDS) – Exposures • 13.3% of all human exposures (#1) • 17.2% of adult exp. (#1) • 9.7% of pediatric (<6y) exp. (#2) – Deaths • Analgesics #1 in fatalities • APAP in combination #5 • APAP alone #8 • ASA alone #13 FDA Advisory Committee – June 30, 2009 • Lowering the MDD of nonprescription APAP for adults, MDD not specified (21 – 6) • Reduce the maximum single adult dose and tablet strength to 650 mg (24 – 13) • 1,000-mg dose be prescription-only (26 – 11) • Eliminating prescription acetaminophen combination products (20 -17) • If APAP-combination prescription drugs stay on the market, they carry a black-box warning (36 – 1) • As of 10/25/10, none of the recommendations have been acted upon http://www.fda.gov/AdvisoryCommittees/Calendar/ucm143083.htm APAP Liquid Preparations • Elixir: 160mg/5mL • Drops: 80mg/0.8mL = 500 mg/5mL APAP Metabolism APAP Metabolism • Primary process is sulfation: high affinity, low capacity • Secondary is glucuronidation: lower affinity, high capacity • P450 pathway (CYP2E1, 1A2, & 3A4) when 1o and 2o saturated. – Generates N-acetyl parabenzoquinone imine (NAPQI) and a semiquinone=reactive species – Ultimately, APAP-mercapturate formed by neutralization of NAPQI w/ glutathione Risk Factors for Liver Injury • • • • Rate of production of NAPQI Amount and production rate of native glutathione Regenerative ability of liver These are all dependent on: – Quantity & rate of APAP absorption – Metabolic activity of CYP2E1, 1A2, & 3A4, rate of elimination/disposition of APAP metabolites, genetics – Nutritional status • Only quantifiable factors are total amount taken and peak levels Triage and Treatment • In acute OD – We use total dose for triage decisions – We use blood level (> 4 h) for treatment • In chronic OD or unknown – We use history and/or lab and/or empirical tx Dose-based Risk • Referral protocols – Poor correlation between history and dose • Pediatric, Acute: > 200 mg/kg • Adult, Acute: > 150 mg/kg • Chronic (<24 hours): • More than 10 gms in 24 hrs in adults • More than 200 mg/kg in 24 hrs in peds • Repeated Supratherapeutic Ingestion (RSI) – Definition: More than maximum rec’d dose for more than 24 hours (4 g/d) – Referred in for lab evaluation Level-Based Risk • Caveats • Only for acute ingestion • Not useful < 4 h • Less useful > 12 h • Cannot be used for chronic ingestion or subacute over > 8 h • May need multiple levels for delayed-release; large amts or if combined with opioids or anticholinergics Caution • No clear cutoff for toxicity – 1.6% to 10% (abstinent alcoholics) risk of hepatotoxicity below 150 mcg/mL @ 4 hr1 – Patients tx w/in 8 hrs w/ IV NAC developed hepatotoxicity in 5.2% of cases2 • Hepatotoxicity = AST or ALT > 1,000 1Ali FM, Boyer EW, Bird SB. Alcohol. 2008 May;42(3):213-8. Epub 2008 Mar 20 2Doyon S, Klein-Schwartz W. Acad Emerg Med. 2009 Jan;16(1):34-9 Glutathione • Glutathione is a tripeptide – Glutamate-Cysteine-Glycine • Need to provide adequate supply of intracellular glutathione in locations of p450 enzymes (liver, kidney) • Other beneficial effects may make it useful in late presenters and hepatic failure from other causes • But does not cross cell membranes N-Acetylcysteine (NAC) • N-actylecysteine (NAC) crosses cell membrane, is de-acetylated, and thus provides intracellular cysteine – Rate-limiting step in the production of intracellular glutathione – If NAC started before depletion of glutathione (~8 h), highly unlikely to see significant hepatotoxity Course of Liver Injury w/o NAC Prevention of Injury Acute Exposure • Time to first NAC dose < 8 hours – Treat if above 150 mcg/mL line – PO / IV protocols w/ equal efficacy – Must have no APAP at end of IV NAC • Acute & > 8 hrs to first NAC dose – IV or PO NAC x 36 hrs from ingestion • Can stop IF non-detectable APAP and no increase in AST/ALT @ end of NAC • Acute ingestion with hepatotoxicity – PO or IV NAC with continuation until clearly improving, transplant or death Prevention of Injury RSI • Often detected serendipitously in ED • Management – IF no measurable APAP (<10 mcg/mL) AND no increased AST/ALT • No treatment – IF measurable APAP • Admit for 24 hrs NAC, recheck APAP/AST/ALT and discontinue if all negative – IF elevated AST/ALT • Treat as for acute presentation with hepatotoxicity Very Late Presenters • Increased survival in patients presenting in fulminant hepatic failure tx w/ IV NAC.1, – Retrospective (n=100): Mortality was 37% in patients who received NAC compared with 58% in patients not given NAC1 – Prospective (n=50): Survival was significantly higher with NAC (48%) than controls (20%)2 – NAC treated patients • Had a lower incidence of cerebral edema (40% v. 68%) • Developed less hypotension requiring inotropic support (48% v. 80%) • Liver function was unchanged 1Harrison PM, Keays R, Bray GP, et al. Lancet 1990;335:1572-1573. 2Keays R, Harrison PM, Wendon JA, et al. BMJ 1991;303:1026-1029. Transplant Referral and Outcome Prognostic Factors • Possible need for transplant (alert team) – Arterial pH < 7.3 after fluid replacement – INR > 6.5 – Serum Cr > 3.4 – Encephalopathy, grade 3 or 4 • Grade 3: Somnolence, confusion, gross disorientation • Grade 4: Coma • During hospital course – Poor • Development of King’s criteria • Persistent elevation of lactate > 12 – Good • Phosphorus < 3.0 • Spontaneously improving INR, ammonia, and mental status What is the Optimal NAC Route? • No class “A” studies and no controlled study has compared IV to PO • PO v. IV efficacy appears equivalent in early presenters • Adverse events – PO: Nausea/vomiting; usually first 1 – 2 doses – IV: Allergic rxns • Minor rxns ~ 14% • Severe ~ 0.1% to 0.3% • Current poison center recs: Use PO unless specific indications for IV or CI to PO – IV indications: Late presentation, >8 h, acidosis, encephalopathy, renal injury, pregnancy, persistent vomiting – PO contraindications: CNS depression, caustics, hydrocarbons, obstruction Proposed UNMH Actions • Acetaminophen Task Force approved the following: – Pharmacy should stock only combination agents with acetaminophen 325mg – Pharmacy should only stock acetaminophen 325mg and maximum single adult dose should be 650mg – Combination agents should be avoided in the inpatient, oxycodone and acetaminophen should be administered as separate agents. – The newly implemented total daily acetaminophen dose calculator should be programmed to fire at 3,200mg. – Over-the-counter combination agents containing acetaminophen should be removed from the formulary – Stock only elixir (160 mg/5 mL) concentration • Plan to obtain input from providers prior to implementation Questions?