* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download New ABPI Code of Practice – what difference will the new
Survey
Document related concepts
Transcript
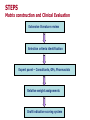
STEPS Dr MIKE SCOTT Chief Pharmacist United Hospitals Trust Antrim Hospital Northern Procurement Ireland Distribution Interest Group (PDIG) 8 June 2006 STEPS (Modified SOJA) selection pharmaceutical economic therapeutic safe Medicines Management “Encompassing the entire way that medicines are selected, procured, delivered, prescribed, administered and reviewed, to optimise the contributions that medicines make to producing informed and desired outcomes of patient care.” Audit Commission 2001 Integrated Medicines Management Decreased length of stay Decreased readmission rate Reduced wastage of patients’ own drugs More accurate drug history Improved appropriateness of medicine use Improved patient safety Improved use of medical and nursing staff time Faster discharge process Improved discharge prescription accuracy Improved medicine use – counselling Improved communication across the interface Tasks Undertaken Communication with primary care on admission Accurate medical history Management of patients’ own drugs Inpatient management including counselling Pharmacist discharge and counselling Communication with primary care on discharge More Accurate Drug History Mean number of queries on the initial inpatient kardex was reduced by 4.2 per patient, reflecting improved medicines management and impacting on length of stay. Improved Medicine Use There was a significant improvement in the medication appropriateness index. Control Intervention Admission Discharge 13.16 9.97 17.48 5.69 Health Service Journal Award 2003 Lack of Integrated Product Use This was identified as a significant deficient element in the current medicines management system. - different choice of agents in a therapeutic class - different generics and parallel imports being used in primary care - confusion for patients, particularly the elderly Cost SECONDARY CARE PROCUREMENT Contract based - 3 years All trusts contribute Tender by generic name Bids assessed by a pharmacist advisory panel Recommendations made to the Executive Panel for approval Executive Committee of Trust Chief Executives Success This contract process has worked well and achieved significant savings for the secondary sector. In the 2004-2007 contract savings in excess of £2 million per annum were achieved representing a 7.6% reduction. 87.2% being subject to good procurement practice as defined by the Audit Commission. Problems Lack of primary care element Loss leading to gain primary care business (80% of spend is in the community) Product name constraint therefore there is no mechanism to consider therapeutically equivalent usage Procurement Primary Care Independent contractors both GP and pharmacist GP prescribes ideally generically Pharmacist supplies cheapest product by procurement Prices set by CSA on tariff Hello! Hello! Northern Health and Social Services Board (NHSSB) Population of 440,000 Three stage process Stage one – branded generics Stage two – therapeutic classes Stage three – generic generics NORTHERN AREA PRESCRIBING FORUM Professional Secretary - Dr M Scott Consultants General Practitioners Community Pharmacists Hospital Pharmacists Board Director of Pharmacy (Chairman) Area Medical Advisor Local Medical Committee Locality Groups for Community Pharmacists Drug and Therapeutics Committee IMPLEMENTATION Phase One Primary Care Prescribing advisors and prescribing support assistants actually promoted and implemented the changes with GPs Secondary Care Consultants agreed to the product switches automatically carried out by pharmacists United gave notice to companies tendering for Regional Contract that NHS price would be a determination in their adjudication – hence different products used THREE YEAR PERIOD Calcichew Adcal Imdur Elantan Adalat LA Isotard XL Gaviscon Peptac Nitrate Patches Nitrodur Proctosedyl Preps Uniroid Preps Diltiazem brands Slozem (once daily) Coracten PRIMARY CARE PERCENTAGES FOR NITRATES Elantan LA 25 -10.4% Elantan LA 50 -17.6% Imdur SR -27.2% Modisal MR -23.5% Monomax SR -31.9% Ismo Retard -58.3 Isotard XL +223.9% STAGE II Therapeutic Tendering Competitive tendering for pharmacologically similar products Current processes use generic name for bidding purposes By default excludes similar chemical entities with the same pharmacological actions and range of potential Eeeny Meeny Miny Mo STEPS selection pharmaceutical economic therapeutic safe STEPS Quality first, then safety, then costeffectiveness Full product integration between primary and secondary care Full ownership by both general practitioners and hospital consultants Standardisation of generics (plus branded) Therapeutic tendering STEPS Selection - agreed criteria (weighted) 1st Step - Clinical Evaluation 2nd Step – Safety Evaluation 3rd Step – Budgetary Impact Assessment Selected medicines for 70% prescribing No restrictions Transparent and Defensible STEPS Improve quality of prescribing information across primary / secondary care Framework updated regularly with emerging evidence Three year formulary (for 70% prescribing will be class specific) No discount into the secondary care sector International Links STEPS ____________________ MATRIX CONSTRUCTION AND CLINICAL EVALUATION STEPS Matrix construction and Clinical Evaluation Extensive literature review Selection criteria identification Expert panel – Consultants, GPs, Pharmacists Relative weight assignments Draft indicative scoring system STEPS Matrix construction and Clinical Evaluation Validation questionnaire (Consultants) Final scoring system for Matrix Matrix sent to all relevant pharmaceutical companies Data analysis Drug entities relative scores ACE INHIBITORS MATRIX Number of licensed indications Number of formulations Trough / peak ratio BP lowering effect Variability in biovailability Interactions Clinical efficacy Side-effects Dosage frequency TOTAL 1000 pts DRUG ENTITIES SELECTION RISK ASSESSMENT A - CRITICAL INFORMATION STEP 1. 2. 3. 4. 5. Labelling Packaging Storage conditions Blisters Patient information leaflets Accept B - ADDED VALUES STEP (EXTRA POINTS) 1. 2. 3. 4. Calendar packs EAN barcode Pack size Tab/cap colouring and marking 5. Label instructions space BUDGET IMPACT ANALYSIS DDD profiling DDD fractions refining Cost calculation both primary and secondary care prices Affordability (selection / budget) FINAL SELECTION OF PRODUCT LINES Therapeutic Classes Completed Statins Proton Pump Inhibitors ACE Is (now being regionalised) July 06 July 06 Oct 06 In progress – ARBs SSRIs Oct 06 Oct 06 Key Requirements COMMUNICATION 80 meetings with key stakeholders Interactive sessions OWNERSHIP – LOCAL Consultants GPs Key Messages 1) Patient care is enhanced 2) Efficiency a) b) Reduced cost to achieve the same quality of patient care Reimbursement of the efficiency to – i. Pay for new expensive treatment modalities ii. Pay for primary care infrastructure, eg CPN DEVELOPMENT Development 1) Regional Steering Group Regional Steering Group Chair - Consultant Clinical Pharmacologist Consultants GPs Hospital Pharmacists Community Pharmacists Prescribing Advisors ABPI representation Decide and oversee the work programme Development 1) Regional Steering Group 2) Regional Pharmaceutical Procurement Unit Regional Pharmaceutical Procurement Unit Regional Procurement Pharmacist 2.5 wte Pharmacists 1 wte Clerical Officer Requiring a Pharmacoeconomic Pharmacist Linked to SGCE and PCEG Development 1) Regional Steering Group 2) Regional Pharmaceutical Procurement Unit 3) Reorganisation of hospital contracts Reorganisation of the Hospital Contracts Coverage of all main therapeutic classes Rolling 3-year contacts rather than one massive contract Regional generic generics contract Development 1) 2) 3) 4) Regional Steering Group Regional Pharmaceutical Procurement Unit Reorganisation of hospital contracts Primary Care aspects Primary Care Aspects Linkage to quality and outcomes framework for new GMS contract Linkage to regional prescribing incentive scheme Linkage to new community pharmacy contract based on quality Linkage to community pharmacy “Managing Your Medicines scheme” (IMM) Development 1) 2) 3) 4) 5) Regional Steering Group Regional Pharmaceutical Procurement Unit Reorganisation of hospital contracts Primary Care aspects Guidance Guidance Assessment relative to indication, eg ACEIs in hypertension in heart failure in diabetic patients Regional guidance NICE ? SMC OTHER APPLICATIONS DRESSINGS Surgical dressings Wound management products Aug 06 FOR BOTH PRIMARY AND SECONDARY CARE BENEFITS Optimised patient care Fully integrated product use in both sectors Selection of product on safety and therapeutic efficiency as the prime determinant Quality at best value for the service Robust, transparent, defensible system of selection Web-based formulary – evidence based Dynamic with regular updates Compliance with EU legislation Matrices created for the different categories Hydrocolloids Silver dressings Logistics assessment Pharmaceutical Clinical Technology 1) Medical and surgical disposables • • Significant involvement of pharmacy in their management Improved management and cost control 2) Point of Care Testing • Managed and controlled by pharmacy via the regional contracting process Pharmaceutical Clinical Technology a) Medical and surgical disposables eg sutures Oct 06 endosurgery Jan 07 endoscopy Jan 07 b) Point of Care Testing - urine testing - blood glucose - misc, eg troponin, BNP, drugs of abuse screen Oct 06 Dec 06 Mar 07 Pharmaceutical Services Improvement Programme (PSIP) Repeat dispensing Minor ailments 28-day dispensing Generic substitution Medicines governance Integrated medicines management Therapeutic tendering Pharmaceutical Clinical Technology