* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Transcript
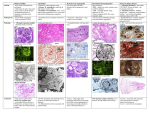
Drugs and The Kidney Drugs & The Kidney Dr. Shahrzad Shahidi Dr. Shahrzad Shahidi Introduction • The heart pumps approximately 25% of CO into the kidneys • Any drug in the blood will eventually reach the highly vascularized kidneys • May potentially cause drug-induced renal failure • The drug may be filtered or secreted into the lumen of the renal tubules • The concentrated drug exposes the kidney tissue to far greater drug concentration per surface area Clinical Presentation • Drug-induced renal disease can mimic renal disease from other causes, such as autoimmune disease & infection • A thorough PEx & medical Hx should be performed • Increase in serum Cr & BUN • Additional urine tests: Pr excretion, Cr concentration, osmolality or Na excretion • A thorough & accurate review of all medications, including all prescription, over-the-counter & herbal medications • Importance of dose & duration of exposure • Rule out all other causes of kidney failure Pseudo Renal Failure • ↑ BUN due to protein catabolism – Steroids, tetracyclines • ↑ SCr due to competitive inhibition of cr secretion – Trimethoprim, Cimetidine • Trimethoprim – 15-35% rise SCr fully expressed after 3 days – More sig in pts with pre-existing renal dysfunction – Can occur with normal doses – Completely reversible when drug is discontinued Mechanisms of nephrotoxin-induced ARF • Direct nephrotoxicity – Tubuloepithelial injury – ATN (e.g.,aminoglycosides) – Osmotic nephrosis (e.g., hypertonic solutions, IV IG) • Interstitial nephritis – Acute allergic interstitial nephritis (e.g., penicillins) – Chronic interstitial nephritis (e.g., calcineurin inhibitors) – Papillary necrosis (e.g., NSAIDs) • Glomerular disease – Glomerulonephritis (e.g., gold, penicillamine, ACE inhibitors) – Renal vasculitis (e.g., hydralazine) • Obstructive uropathy – Crystalline nephropathy (e.g., acyclovir, indinavir) • Indirect nephrotoxicity – Decreases intrarenal blood flow (e.g., ACE inhibitors, NSAIDs) Patterns of Drug-induced Lesions Tubulointerstitium • Acute tubular injury - Osmotic nephrosis - Nephrocalcinosis - Crystal NP • Acute interstitial nephritis Glomeruli • Minimal change disease Blood vessels • Hyalinosis • Thrombotic • Focal segmental glomerulosclerosis micro-angiopathy • Vasculitis • Membranous GN • Crescentic GN • Chronic tubulointer- • Thrombotic microstitial nephropathy angiopathy Patterns of Drug-induced Lesions Tubulointerstitium Glomeruli Acute interstitial Antibiotics NSAID nephritis ACE-I Diazepam Chronic Lithium tubulointerstitial nephropathy Thiazids COX2-I CNI tubular injury Acute Minimal change Lithium NSAID disease Virostatics - Osmotic nephrosis HES Bisphosphonates - Nephrocalcinosis Ranitidin - Chrystal NPCisplatin Barbiturates Quinolones Sirolimus Focal segmental Interferon glomerulosclerosis Bisphosphonates Captopril Hydralazin Membranous GN Crescentic GNRifampicin CNI Thrombotic microCisplatin Tamoxifen angiopathy Blood vessels Hyalinosis CNI ThromboticClopidogrel microSulfasalazine angiopathy Vasculitis Quinine Phenytoin Case PP: Female , 50 y CC: Fatigue since 1 wk ago PI: Nocturia, Polyuria 2 wks PH: Sinusitis 3 wks ago, treated with Amoxicilline 500 mg 3 tab/d for 2 wks, HTN 5 yrs FH: HTN in her mother, DM in her brother PE: BP: 90/60, PR: 86, Pallor, dry mouth & skin. Amoxicilline Nocturia, Polyuria Fatigue Case • • • • • • • Hb: 10 g/L FBS: 80 mg/dl BUN: 60 mg/dl Cr: 4 mg/dl Na: 124 meq/L K: 6 meq/L UA: 9 mg/dl • U/A: – SG 1.007 – Pr + – Glu + – RBC 6-8/HPF – WBC 10-15/HPF – WBC cast 0-1/LPF • U/C: Neg Based on Experimental AIN www.nature.com/ki/journal Pre-renal causes • Vasoconstriction – Contrast agents – Amphotericin, noradrenalin, immunosuppressive agents such as tacrolimus & cyclosporine – Iodinated contrast media, in particular, have been shown to inhibit the synthesis of nitric oxide in renal artery smooth muscle Radiocontrast Agents Ionic vs. Nonionic High (1500-1800) Low (600-850) Iso-osmolal (~ 290 mOsm/kg)) Radiocontrast Agents • Pathogenesis: – Renal Vasoconstriction (Adenosine, Endothelin) – Tubular Injury Oxidative stress induced damage Radiocontrast Agents • Risk Factors: – Underlying renal disease (Cr >1.5mg/dl) – Diabetic nephropathy, HF, Hypovolemia – Multiple Myeloma – Dose (lower doses safer but not necessarily safe) Radiocontrast Agents • Incidence – Negligible when renal function is normal (even if diabetic) – 4 -11% in patients with Cr 1.5 – 4.0 mg/dL – 50% if Cr > 4.0 mg/dL and in diabetic nephropathy • Diagnosis – Characteristic rise in plasma Cr following administration of the agent – Radiocontrast Agents Prevention: – Use of alternative diagnostic procedures in high risk patients – Avoidance of volume depletion or other nephrotoxins – Low-doses of low- or iso-somolar agent – IV saline Case • 65 year old male with H/o HTN, ventricular arrythmias controlled on Amiodarone, OA on NSAIDs. presents with puffiness on face on waking up. Has bilateral pitting edema. • U/A: 3+ pr, RBC 3-5/HPF, WBC 15-20/HPF • 24 h urine pr : 4 g • BUN: 80 mg/dl , Cr: 5 mg/dl , Serum Albumin : 2.8 g/dl, TSH : Nr • The most likely Diagnosis? A) Amiodarone induced hypothyroidism B) RPGN C) NSAIDs induced nephrotic syndrom & interstitial nephritis • The most likely Management & Follow up? Nephrotic syndrome • Abnormal amounts of Pr in the urine • Drugs : NSAIDs, penicillamine & gold,…. • Damage the glomerulus & alter the ability of the glomerulus to prevent Pr from being filtered • Stopping the drug may resolve the damage to the glomerulus Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Chemical Structure Generic Name Acetic acids: Fenamates: Diclofenac, Indomethacin, Sulindac, Meclofenamate, Mefenamic acid Napthylalkanones: Nabumetone Oxicams: Meloxicam , Piroxicam Propionic acids: Fenoprofen, Flurbiprofen, Ibuprofen, Ketoprofen, Naproxen, Oxaprozin Pyranocaboxylic acid: Etodolac Pyrrolizine carboxylic acid: Ketorolac Selective COX-2 inhibitors: Celecoxib, Rofecoxib NSAIDs • Hemodynamically- Induced ARF • Acute Interstitial Nephropathy + Proteinuria • Papillary necrosis & CRF(Analgesic nephropathy) • Salt & water retention: Hyperkalemia, HTN NSAIDs • Acute Interstitial Nephropathy + Proteinuria Acute interstitial nephritis Minimal-change glomerular disease Proteinuria Prognosis good after discontinuation of therapy; Corticosteroids ? NSAIDs • Analgesic nephropathy (Chronic Interstitial Nephritis & Papillary necrosis ) – Single vs. combined analgesics – Dose dependent (at least 1 kg) – Patients with history of depended behaviors – Slowly progressive ; Asymptomatic, sometimes hematuria, flank pain, or urinary infections. – Being responsible for 1% to 3% of ESRD cases Analgesic Nephropathy Papillary necrosis Analgesic Nephropathy Papillary necrosis NSAIDs/COX II Inhibitors • Physician would like to switch previous patient from Naproxen to Celecoxib • Are Cox II inhibitors less likely to cause ARF compared to NSAIDs? NSAIDs/COXibs • Use with caution in CKD (grade 3 or greater) • Inhibit renal vasodilatory prostaglandins E2 & I2 – Produced by COX-2 • Reversible reduction in GFR – Higher risk if intravascular volume depletion – Management: D/C drug, use alternate analgesia • HTN – Edema, sodium and water retention – Mean increase SBP 5 mm Hg • Hyperkalemia Risk – Blunting of PG-mediated renin release Osmotic nephrosis • A morphological pattern with vacuolization & swelling of the renal proximal tubular cells. • The term refers to a nonspecific histopathologic finding rather than defining a specific entity. • It has a broad clinical spectrum that includes AKI & CKD in rare cases. • High doses of mannitol, soucrose-containing IVIg, contrast dye , dextrans & starches are nephrotoxic • Mechanism: uptake of these large molecules by pinocytosis into the proximal tubule cells. Post-renal failure • Usually results from a mechanical barrier to moving urine from the collecting tubules into the bladder • Mechanical obstruction : – Bladder retention (in BPH, Neurogenic bladder) – Kidney stones – Drugs that precipitate in the kidney (acyclovir, ganciclovir) DRUGS OF ABUSE • Cocaine & heroin • Cocaine use can cause renal artery thrombosis (clotting), severe HTN & interstitial nephritis • Long-term cocaine use can lead to CRF • Tobacco use increase the progression rate of CKD • Long-term tobacco use also increases the risk of kidney cancer Crystal-Induced ARF • Acyclovir (antiviral agent ) • Indinavir (antiretroviral agent, protease inhibitor) • Methotrexate (antineoplastic agent, antimetabolite) • Sulfonamide antibiotics • Triamterene Crystal-Induced ARF Sulfonamide crystals Indinavir sulfate urinary crystals Gagnon et al. 1998, Ann Intern Med 128-321 Case • 52 yo male with Type 2 DM • Baseline cr 1.8 mg/dl; BP 145/90 • Enalapril 10 mg daily started & 2 weeks later: BP 135/80 • Serum cr 2.2 mg/dl Optimal Use of ACEI/ARB • Cr ↑ 1.8 to 2.2 mg/dl in 2 wks – Accept 20-30% increase in serum cr within 1-2 months of initiation • In fact, this could be an indication that the drugs are exerting their desired actions to help preserve renal function • Check serum cr 1-2 wks after initiation, then in 2-4 wks • If > 30% change, decrease ACEI/ARB dose by 50% & repeat Ser Cr in 4 wks (exclude hypovolemia/NSAIDs, etc) • If > 50% rise in Ser Cr – rule out RAS • Repeat serum cr in this patient in 1-2 wks to ensure it has stabilized Case • 82 yo female with osteoarthritis • Admitted to hospital for CAP & dehydration • Meds: Losartan 100mg daily + Naproxen 250mg BID • Cr 3 mg/dl Optimal Use of ACEI/ARB • Cr on admission 3 mg/dl in patient with CAP & dehydration – Discontinue NSAID & hold ARB until infection treated & patient is rehydrated/cr reduced • Resume ARB & monitor serum cr Causes of AKI after Initiation of Therapy with ACE Inhibitor or ARB • BP insufficient for adequate renal perfusion – Poor cardiac output – Low systemic vascular resistance (e.g., as in sepsis) – Volume depletion (GI loss, excess diuretic use, …) • Presence of renal vascular disease* – Bilateral renal artery stenosis – Stenosis of dominant or single kidney – Afferent arteriolar narrowing (caused by HTN, cyclo..) – Diffuse atherosclerosis in smaller renal vessels • Vasoconstrictor agents (NSAIDs, cyclosporine) Prevention: General Rules • Be aware of nephrotoxic potential of specific drugs • Identify patients at risk • Be aware of increased risk in elderly • Asses the benefit/risk ratio for Rx of potentially nephrotoxic drug • Monitor the RFT if necessary Prevention: General Rules (Cont’d) • Avoid dehydration • Limit dose & duration of treatment • Adjust the dose based on changes in GFR • Avoid a combination of potentially nephrotoxic drugs Conclusion • Many drugs cause AKI • Increase the risk of drug-induced AKI: – Age (particularly over 65 years) – Pre-existing renal impairment – Comorbidities such as DM, HF, liver cirrhosis – Hypovolaemia • Addressing potential risk factors • Understanding of the mechanisms of nephrotoxicity involved