* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download sOLUBILITY
Survey
Document related concepts
Transcript
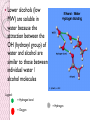
Experiment 10 Group 6 Ken Caceres Christian Daroya Rubycor Duran Jomari Galecio Jessa Pilorin BSBIO1A Objectives: 1. 2. 3. To define solubility To identify the factors affecting it To use the solubility class diagram competently Solubility It describes the amount of one substance (solute) that will dissolve in a specified amount of another substance (solvent) under stated conditions Solute - A minor component of a solution. Solution - A homogeneous mixture. Solvent - The major component of a solution. Soluble – that can be dissolved Ex. Ethanol and water Insoluble – cannot be dissolved Slightly soluble - A very small amount will dissolve and the solution will be transparent ex. Pentane and water Solubility Phenomenon: Like dissolves like Polar solute dissolves polar solvent Ex. NaCl is soluble in water Nonpolar solute dissolves nonpolar solvent Ex. Pentane and benzene Factors 1. Van der Waals forces 2. Dipole forces between polar molecules 3. Hydrogen bonding 4. Branching in some compounds 5. Functional groups 1.Van der Waals Forces (VWF) These are forces between 2 nonpolar compounds Non-polar liquids dissolve to each other because of VWF Ion-induced dipole attraction a type of VWF which is a weak attraction that results when the approach of an ion induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. 2. Dipole – dipole interaction Forces between 2 polar compounds Cause of polar liquid’s solubility to each other Positive and negative ends attract each other 3. H Bonding these occur between polar covalent molecules that possess a hydrogen bonded to an extremely electronegative element, specifically - N, O, and F Lower alcohols (low MW) are soluble in water because the attraction between the OH (hydroxyl group) of water and alcohol are similar to those between individual water / alcohol molecules Legend: = Hydrogen bond = Oxygen = Hydrogen Increase in carbon chain length, in highmolecular weight alcohols, decreased water solubility This is because the strength of VWF increases with the increasing chain length Branching in some compounds favors solubility in water Because branched carbon chains will not allow close approach of the branched molecules Thus leading to a decreased VWF and an increased H bonding Functional groups Solubility in water indicates the presence of OH group Solubility in a basic solvent (NaHCO3) indicates the presence of an acidic functional group Ex. Solubility of benzoic acid in NaHCO3 with –COOH (carboxyl group) as functional group. Solubility Class of Organic Compounds = determines the possible functional group classes to which the unknown may belong S1 These are very polar compounds which consist of salts of carboxylic acids or amines. It is also possible the compound is of low molecular weight and has many polar functional groups such as a carbohydrate. Low mol. Wt. (MW) amines S2 These compounds are low molecular weight (generally less than 5 carbons) with a polar functional group such as carboxylic acid, amine, alcohol, aldehyde, or ketone. Low MW carboxylic acids SOLUBILITY TEST ANALYSIS: A1 Higher molecular weight carboxylic acids fall into this class. Strong acids A2 Phenols show this kind of solubility. Weak acids SOLUBILITY TEST ANALYSIS: B1 Primary, secondary and tertiary amines fall into this class. However, if there are two or more phenyl groups on the nitrogen, the amine will probably not be basic enough to form the salt and will, then, be insoluble. N1 These are higher molecular weight compounds (generally more than 9 carbons) containing an oxygen atom. SOLUBILITY TEST ANALYSIS: N2 These are medium size molecules (generally containing from 5 to 9 carbons) containing an oxygen atom. INERT These are neutral compounds. Alkyl halides and alkanes fall into this class. SOLUBILITY TEST ANALYSIS: Test Compounds Urea Molecular Structural Formula Formula O CO(NH2)2 C H2N NH2 Common name Properties MW: 60.06g/mol Carbamide Appears as a resin white solid Isourea MP:133135°C Urea Distilled H2O soluble Ether Insoluble NaOH Soluble NaHCO3 Soluble HCl Soluble H2SO4 Soluble H3PO4 soluble Solubility Class S2 Benzoic acid IUPAC Name Common Name Molecular Formula Benzoic acid Benzenecarboxylic acid C7H6O2 Structural Formula Benzoic acid and distilled water Insoluble to cold water but can be soluble to hot water Water-solubility of carboxylic acids decrease as the number of carbons increase. In case of benzoic acid, the molecule contains seven carbons and six of them form a benzene ring. Benzoic acid and Ether Soluble Benzoic acid is soluble in ether due to the low polarity of its molecules and weak intermolecular forces. Benzoic acid and NaOH Soluble NaOH helps deprotonate benzoic acid organic acids (such as carboxylic acids) react with bases to form water soluble salts Either strong/weak acid R-COOH + Na+ + OH- + H2O → R-COO- Na+ + OH- + H3O+ Benzoic Acid and NaHCO3 soluble NaHCO3 is a weak base that reacts with organic acids to form water soluble salts Solubility to NaHCO3 makes it a strong acid or Class A1 Benzoic Acid and HCl Insoluble HCl cannot dissolve benzoic acid because it is a nonpolar compound. Benzoic acid can’t loose a proton (H+) just like HCl Benzoic Acid and H2SO4 Insoluble H2SO4 cannot dissolve benzoic acid because it is a nonpolar compound. Benzoic acid can’t loose a proton (H+) just like H2SO4. Benzoic Acid and H3PO4 Insoluble H3PO4 cannot dissolve benzoic acid because it is a nonpolar compound. Benzoic acid can’t loose a proton (H+) just like H3PO4