* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Incorporating a Fingerprinting System into the
Survey
Document related concepts
Transcript
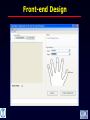
Incorporating a Fingerprinting System into the Western Kenya Health and Demographic Surveillance System, 2009 Ezekiel Chiteri, Wilfred Ijaa Frank Odhiambo, Marta Ackers, Allen Hightower, James Kwach, Kayla Laserson INDEPTH CONFERENCE 27th October 2009 KEMRI/CDC Research and Public Health Collaboration Kisumu, Kenya Background(1) • KEMRI/CDC has multiple projects – Health and demographic surveillance System (HDSS), malaria, TB and HIV research • Projects assign their participants unique study IDs – Some projects distribute ID cards • Projects operate in same study area, same study pop • Individuals can enroll in one or more projects – Some projects do not allow cross-study participation • Health facility (HF) surveillance conducted as follows – In-patient conducted in 1 hospital – Out-patient conducted in 3 clinics – HIV and TB care and treatment programs conducted in all health facilities • The current method of linkage between HDSS and HF/projects is through a search engine tool Background (2) “SINGLE” POPULATION KEMRI/CDC & OTHER MEDICAL HEALTH ORGANIZATIONS Background (3) Challenges to linking individuals from HDSS data to HF/ studies information • • • • Misplaced ID cards Name similarities Migrations reconciliation Non uniform identification of participants by different projects/studies Objective To develop an efficient identification system for the whole organization: • To be used for linking the HDSS to all HF/projects’ data • Scalable and adaptable • Acceptable • Cost effective Methodology • Design a fingerprint system – Database design – Selection of hardware and software development kits (SDKs) – User application design • Develop SOPs and procedures for the fingerprint system • Ethical clearance -obtained from the KEMRI Ethical Review Committee and the CDC Institutional Review Board • Implementation of the fingerprint system • Post implementation review Database Design Front-end Design System Specification (HARDWARE) • Finger Print Readers – Microsoft Fingerprint Reader – Digital persona • Computers – Pentium Processor (i386) (2.0 GHz or later) – 1GB RAM or more – 5GB of free space in the hard disk. System Specification (SOFTWARE) • Database and SDK – Fingerprint SDK 2009 for Windows by GriauleBiometrics – MS SQL SERVER 2005 • Operating System – Windows XP Professional System Costs Item Recommended Brand Fingerpt Reader Microsoft fingerprint reader Fingerprint SDK 2009 by SDK griaulebiometrics Any Brand with the above PC specifications Average Cost Per work Station Average Cost $50 $36 $1200 $1286 Implementation STEPS: • System deployment and user training • Health facility and additional study sites’ data collection points • HDSS household surveillance data collection points • Fingerprint data consolidation Fingerprint Collection Points POPULATION POINT OF OPERATION TOOLS Health facility Mobile Surveillance PC, Laptop, Tablet PC Other studies Fingerprint Reader Data Point 4 Data Point 5 Fingerprints Centralized or Replicated Database Results • 868 fingerprints collected • 1352 patient visits recorded in the hospitals • Patient visits include multiple visits by the individuals – Fingerprints are enrolled only once – Enrolled fingerprints used to identify individuals in subsequent visits • Children<1 were not fingerprinted Limitations • Children under 1 year were not finger printed due to a low success rate in enrolling their finger prints during the pilot stage Conclusions • High acceptability at current collection points • Feasible means of individual identification in health and demographic surveillance research • It takes short time to enroll/identify individuals What next? • Measuring the success rate of fingerprint identification • Build fingerprint database of all residents using HDSS surveillance • Measure acceptability in our surveillance area Acknowledgements • Colleagues (Programmers) • DSS data managers and field workers • Study participants • KEMRI/CDC • PEPFAR