* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Large Vestibular Aqueduct Syndrome: A Case Study
Survey
Document related concepts
Sound localization wikipedia , lookup
Telecommunications relay service wikipedia , lookup
Olivocochlear system wikipedia , lookup
Evolution of mammalian auditory ossicles wikipedia , lookup
Lip reading wikipedia , lookup
Auditory system wikipedia , lookup
Hearing loss wikipedia , lookup
Hearing aid wikipedia , lookup
Noise-induced hearing loss wikipedia , lookup
Sensorineural hearing loss wikipedia , lookup
Audiology and hearing health professionals in developed and developing countries wikipedia , lookup
Transcript
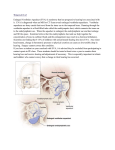
J Am Acad Audiol 16:822–828 (2005) Large Vestibular Aqueduct Syndrome: A Case Study Jackie L. Clark* Ross J. Roeser* Abstract A 23-month-old female was referred for hearing aid fitting after failing newborn hearing screening and being diagnosed with significant hearing loss through subsequent diagnostic testing. Auditory brainstem response (ABR) and behavioral testing revealed a moderate-to-severe bilateral mixed hearing loss. Prior to the hearing aid evaluation, tympanostomy tubes had been placed bilaterally with little or no apparent change in hearing sensitivity. Initial testing during the hearing aid fitting confirmed earlier findings, but abnormal middle ear results were observed, requiring referral for additional otologic management. Following medical clearance, binaural digital programmable hearing aids were fit using Desired Sensation Level parameters. Behavioral testing and probe microphone measures showed significant improvements in audibility. Decrease in hearing sensitivity was observed six months following hearing aid fitting. Radiological studies, ordered due to the mixed component and decreased hearing sensitivity, revealed large vestibular aqueduct syndrome (LVAS). Based on the diagnosis of LVAS, a cochlear implant was placed on the right ear; almost immediate speech-language gains were observed. Key Words: Amplification, aural habilitation, cochlear implantation, large vestibular aqueduct syndrome, pediatric audiology Abbreviations: BKB = Bamford-Kowal-Bench; DSL = Desired Sensation Level; LVAS = large vestibular aqueduct syndrome; MRL = minimal response levels; SOPCHL = sudden onset or progressive childhood hearing loss; WDRC = wide dynamic range compression Sumario Una niña de 23 meses de edad fue referida para adaptación de audífonos luego de fallar una prueba de tamizaje auditivo para recién nacidos, y de establecerse que era portadora de una hipoacusia significativa con el uso de pruebas diagnósticas subsecuentes. Las respuestas evocadas de tallo cerebral (ABR) y las pruebas audiométricas conductuales demostraron una hipoacusia mixta bilateral de grado moderado a severo. Previo a la evaluación para adaptar auxiliares auditivos se le colocaron tubos timpánicos sin cambio aparente su sensibilidad auditiva. Las pruebas iniciales durante la adaptación de los auxiliares auditivos confirmaron los hallazgos iniciales, pero se observaron resultados anormales en la función del oído medio que requirieron una referencia para manejo otológico. Cuándo fue dada de alta médicamente, se le adaptaron auxiliares auditivos digitalmente programables en forma binaural utilizando parámetros de Nivel Deseado de Sensación. Las pruebas conductuales y las medidas de micrófono de sonda mostraron un incremento significativo en la audibilidad. Una disminución en la sensibilidad auditiva se observó seis meses después de la adaptación de los audífonos. Los estudios radiológicos, ordenados debido al componente mixto y a la disminución en la sensibilidad auditiva, revelaron un síndrome de acueducto vestibular grande (LVAS). Basados en el diagnóstico de LVAS se colocó un implante coclear en el oído derecho, y casi de inmediato, se observó una mejoría en el hablalenguaje. *UT Dallas/Callier Center for Communication Disorders Jackie Clark, Ph.D., UT Dallas/Callier Center, 1966 Inwood Road, Dallas, TX 75235; Phone: 214-905-3031; E-mail: [email protected] 822 Large Vestibular Aqueduct Syndrome/Clark and Roeser Palabras Clave: Amplificación, habilitación aural, implante coclear, síndrome de acueducto vestibular grande, audiología pediátrica Abreviaturas: BKB = Bamford-Kowal-Bench; DSL = Nivel Deseado de Sensación; LVAS = síndrome de acueducto vestibular grande; MRL = niveles de respuesta mínima; SOPCHL = Hipoacusia de inicio súbito o progresiva de la infancia; WDRC = compresión de rango dinámico amplio D etermining etiology, prognosis, and management for sudden onset or progressive childhood hearing loss (SOPCHL) remains somewhat controversial and unknown. Retrospective studies using sequential audiograms suggest that it is impossible to analyze critical variables with any degree of validity. However, two possible prognostic elements are audiogram morphology and the degree of initial hearing loss (Molini et al, 1998). While there are multiple etiologies associated with SOPCHL, the single most prevalent is Large Vestibular Aqueduct Syndrome (LVAS), accounting for 15–20% of the cases (Emmett, 1985; Arcand et al, 1991). Valvassori and Clemis (1978) first described radiographic observations for 50 patients with LVAS and documented the size of the aqueduct in these patients ranging from 1.5–8.0 mm in diameter (normal diameter is defined as less than 1.5 mm), with bilateral involvement being twice as prevalent. In children, LVAS is associated with asymmetrical, sudden, or progressive mixed or sensorineural hearing loss, typically with onset within the first few years of life. Children with LVAS often experience decreased hearing following minor trauma, infection, or activities involving a Valsalva maneuver (Goeverts et al, 1999; Can et al, 2004). LVAS is more prevalent in females than males and is often associated with inner ear anomalies such as hypoplastic cochlea, congenital disorders (e.g., CHARGE, Alagille syndrome, Pendred syndrome, branchiooto-renal syndrome) or a variant of MondiniAlexandre dysplasia (Arcand et al, 1991; Temple et al, 1999). An occasional finding of vestibular complications can be found with LVAS (Madden et al, 2003). LVAS is the most common radiographically detectable malformation found in children. It typically coexists with other anatomical structural abnormalities in patients with early onset of hearing loss (Okumura et al, 1995). Histopathology sections of enlarged human vestibular aqueducts demonstrate thin-walls lined with flattened epithelium without the normal architecture of papillary folds (Harnsberger et al, 1995). It appears that LVAS results from an arrest in development during the fifth week of gestation, prior to a narrowing of the vestibular aqueduct (Jackler and De La Cruz, 1989). In this paper, the audiological management of a toddler referred for a hearing aid evaluation and subsequently diagnosed with LVAS is described. LVAS has been well documented in the medical and radiological literature, but little has been published in audiological journals. CASE REPORT A 23-month-old toddler was referred to the UT Dallas/Callier Center for Communication Disorders from a nearby medical facility for a hearing aid evaluation. This youngster had several previous diagnostic ABRs and pediatric behavioral audiometric evaluations. Birth and medical history were unremarkable, with no known family history of hearing loss. Diagnostic ABR evaluation, six and a half months following a failed newborn hearing screening, showed 70 and 80 dB nHL thresholds using clicks and tone bursts (500 and 4000 Hz) for the right and left ears, respectively. A 40 dB nHL unmasked bone-conduction threshold was obtained using click stimuli. Subsequent diagnostic ABR evaluations indicated fluctuations between 50 and 80 dB nHL for clicks and tone bursts and consistent 40–45 dB nHL unmasked bone-conduction click thresholds (see Figure 1a and 1b). Behavioral audiometric minimum response levels (MRL) in sound field using visual reinforcement audiometry, completed when the patient was 23 months old, revealed warble tone and speech awareness thresholds at 50–70 dB HL. Unmasked pure-tone bone-conduction and speech-awareness thresholds were present at 30 dB HL. Tympanograms varied significantly over time from Type “B” with 823 Journal of the American Academy of Audiology/Volume 16, Number 10, 2005 Figure 1. Audiometric data obtained at five months showing a severe bilateral hearing loss: (a) click-evoked ABR thresholds for each ear; (b) click-evoked latency-intensity functions for each ear and a bone-conduction response at 45 dB nHL; (c) 500 Hz tone-evoked ABR responses for each ear. both high and low C1 values, “A,” “As,” “C,” and borderline “A”/“As,” with absent ipsilateral reflexes. The child’s otolaryngologist suspected a middle ear pathology, and tympanostomy tubes were placed bilaterally, but she was cleared for binaural hearing aid fitting. Simultaneously, an MRI was ordered to investigate the abnormal middle ear findings, as well as the suspected fluctuating hearing loss. Due to the fluctuating status of hearing loss and the aggressive medical and 824 audiological monitoring, wide dynamic range compression (WDRC) digital behind-the-ear hearing aids were programmed using the SP3 portable programmer (DSL i/o) and dispensed binaurally. Both simulated probe microphone (Figure 2a, 2b) and electroacoustic measures validated programming parameters and level of amplification. Verification of fit was established (AudioScan RM500) while each hearing aid was placed in the test box, and using three simulated input parameters with Large Vestibular Aqueduct Syndrome/Clark and Roeser a. Right Ear Estimated SPL in dB at TM 140 120 100 Gain MPO Target Gain 80 Dynamic a Range R g of o Soft S t Sounds o Threshold 60 40 20 250 500 1000 2000 4000 8000 Frequency in Hz Left Ear b. Estimated SPL in dB at TM 140 120 100 Gain MPO 80 Target Gain Threshold Dynamic a Range R g of o Soft S t Sounds o 60 40 20 250 500 1000 2000 4000 8000 Frequency in Hz Soundfield Audiometry c. Hearing Level in dB 0 -20 -40 -60 -80 -100 Aided -120 0 0 k k k k k k k 25 50 1 1.5 2 3 4 6 8 Frequency in Hz SAT = 35 dB HL Figure 2. Verification of fit using real-ear and behavioral soundfield aided thresholds at two years of age. Real-ear performance was verified (AudioScan RM500) while each hearing aid was placed in the test box and using three simulated input parameters (soft, normal, and loud) with age predicted real-ear-to-coupler differences. Verification of fit using the test box and simulated mode became necessary since the patient was unable to tolerate in situ probe microphone measurements. Probe microphone measures were essentially the same for each ear: (a) right ear, (b) left ear. Aided soundfield thresholds at 500 Hz and 2000 Hz (c) confirmed significant improvement in threshold sensitivity. Figure 3. Pre-implant audiometric data showing (a) severe/profound bilateral mixed hearing loss; (b) binaural aided thresholds showed significant benefit through 2000 Hz 825 Journal of the American Academy of Audiology/Volume 16, Number 10, 2005 Figure 4. Soundfield thresholds obtained following cochlear implantation. age predicted real ear to coupler differences. Verification of fit using the test box and simulated mode became necessary since the patient was unable to tolerate in situ probe microphone measurements. Simulated probe microphone measures confirmed that the Maximum Power Output did not exceed the toddler’s predicted Uncomfortable Loudness Levels for each ear (Figure 2a, 2b). Minimal response levels for binaurally aided behavioral sound field using warble tones and speech stimuli demonstrated a significant improvement in hearing status (Figure 2c). The toddler’s mother was counseled on the care and use of the instruments and advised to return in 30 days for a follow-up hearing aid check. Upon returning for the scheduled 30-day follow-up hearing aid check, the medical diagnosis of bilateral LVAS and mild cochlear dysplasia was made. At the time of the hearing aid check, parental subjective observation of the toddler’s auditory behavior indicated an apparent significant improvement in audibility when hearing aids were worn, and no complications with the instruments were noted. Electroacoustic analysis of both instruments verified appropriate function. Six months following the hearing aid fitting, ear specific behavioral play audiometry showed progression of the hearing loss to severe to profound with a 35–55 dB conductive component, as well as decreased high-frequency aided benefit (Figure 3). Based on the progression of hearing loss that is expected to accompany LVAS, this toddler was considered an appropriate cochlear implant candidate. A multichannel cochlear implant (Med El Combi 30+) was implanted in the right ear ten months post–initial evaluation without complications, and stimulation was successfully initiated one month later. Ear choice for cochlear implantation was made by criteria determined by the otolaryngologist at the medical facility where implantation was conducted. A two month post–cochlear implant follow-up audiological evaluation revealed significant improvement in soundfield awareness with the implant (Figure 4). The toddler was enrolled in the UT Dallas/Callier Center Preschool oral program. Table 1 shows pre-implant and twomonth postimplant speech-language test results. As shown, significant gains were seen in all four tests administered. Parental and school reports were consistent with test scores. The prognosis for functional auditory and speech/language skills for this youngster appears to be very positive. DISCUSSION AND CONCLUSIONS T his paper describes findings for a young child with a severe bilateral fluctuating Table 1. Speech-Language Evaluation Results, Pre- and Postimplant Pre-Implant 11/03 Postimplant 4/04 Early Speech Perception Test (ESP) 2/12 8/12 Category 2 Infant-Toddler Meaningful Auditory Integration Scale (IT-MAIS) 9/40 27/40 Spontaneously respond to environmental sounds? Rarely Always Glendonald Auditory Screening Procedure (GASP) Words CNT Approximates phonemes 826 Large Vestibular Aqueduct Syndrome/Clark and Roeser mixed hearing loss diagnosed with LVAS. Hearing aid fitting provided adequate benefit initially, but the progressive nature of the hearing loss indicated that traditional amplification would not allow for long-term benefit for this child. Based on the probability of decreased hearing sensitivity, a cochlear implant was placed on the child’s right ear, and significant improvement in sound awareness and speech and language performance was observed within a twomonth period. Despite sensorineural hearing loss being most prevalent in LVAS, a conductive component may occur in some individuals, such as the case reported here. Tympanometric findings may or may not be normal in the presence of a mixed hearing loss. It is believed that the conductive finding can be the result of decreased mobility of the stapes resulting from increased perilymphatic pressure, stapes fixation, or an incomplete transmission of the ossicles/ossicular discontinuity because of incomplete bone formation around the inner ear (Valvasorri, 1983; Shirazi et al, 1994; Nakashima et al, 2000). Due to the apparent conductive component in some of the LVAS children (both with an air-bone gap in the absence or presence of Type “B” tympanograms), a medical misdiagnosis of otitis media with effusion can easily delay the eventual finding of LVAS. Without the cooperative interaction between the otolaryngologist, audiologist, and radiologist, the finding of LVAS becomes very difficult to make in such a timely manner as in this particular case. A variety of theories have been postulated to explain the progressive sensorineural hearing loss component found with LVAS. Some believe that architectural changes result in a dysfunctional regulating mechanism or utriculoendolymphatic valve for endolymph electrolytes causing an accumulation of toxic by-products or fluid reflux within the inner ear (Jackler and de La Cruz, 1989; Levenson et al, 1989; Pyle, 2000). Others suggest an abnormally patent endolymphatic system is more vulnerable to sudden fluctuations in cerebral spinal fluid (Arenberg, 1980) or that there is a weakness at the level of the membranous cochlear partitions leading to rupture following sudden pressure changes (Jackler et al, 1987). The finding that the child in this report received significant benefit from a cochlear implant was not necessarily unexpected. Au and Gibson (1999) reported results from ten children with LVAS who received multichannel cochlear implants. Electrode insertion for these children was uneventful, with the exception of an initial gush of perilymph when the otic capsule was opened in seven ears, and no postoperative complications were observed. Within six months, the average Bamford-Kowal-Bench (BKB) sentence recognition scores improved from 31–79%. Positive outcomes were also reported by Temple et al (1999) for seven patients (five adults and two children) diagnosed with LVAS who received cochlear implants. It appears that the outcome observed with this patient and also by Temple et al (1999) suggest that patients with LVAS should be considered as candidates for cochlear implants even if their hearing sensitivity is not in the severe-to-profound range, based on the prognosis of the progressive hearing loss. Since LVAS is associated with sudden or progressive hearing loss, typically with onset within the first few years of life, it is possible for an infant to “pass” a universal newborn infant hearing screening yet acquire hearing loss shortly thereafter. This observation reinforces the need for followup audiological testing when infants are felt to be delayed in sound awareness and/or speech and language skills. REFERENCES Arcand P, Desrosiers M, Dube J, Abela A. (1991) The large vestibular aqueduct syndrome and sensorineural hearing loss in the pediatric population. J Otolaryngol 20(4):247–250. Arenberg IK. (1980) Abnormalities, congenital anomalies and unusual anatomic variations of the endolymphatic sac and vestibular aqueduct: clinical, surgical, and radiographic correlations. Am J Otolaryngol 2:118–149. Au G, Gibson W. (1999) Cochlear implantation in children with large vestibular aqueduct syndrome. Am J Otol 20:183–186. Can K, Gocmen H, Kurt A, Samim E. (2004) Sudden hearing loss due to large vestibular aqueduct syndrome in a child: should exploratory tympanotomy be performed? Int J Pediatr Otorhinolaryngol 68(6):841–844. Emmett J. (1985) The large vestibular aqueduct syndrome. Am J Otol 6:387–403. Goeverts PJ, Casselman J, Daemers K, deCeulaer G, Somers T, Offeciers FE. (1999) Audiological findings 827 Journal of the American Academy of Audiology/Volume 16, Number 10, 2005 in large vestibular aqueduct syndrome. Int J Pediatr Otorhinolaryngol 51(3):157–164. Harnsberger HR, Dahlen RT, Shelton C, Gray SD, Parkin JL. (1995) Advanced techniques in magnetic resonance imaging in the evaluation of the large endolymphatic duct and sac syndrome. Laryngoscope 105:1037–1042. Jackler RK, de La Cruz A. (1989) The large vestibular aqueduct syndrome. Laryngoscope 99:1238–1242. Jackler RK, Luxford WM, House WF. (1987) Congenital malformations on the inner ear: a classification based on embryogenesis. Laryngoscope 97:2–14. Levenson MJ, Parieier SC, Jacobs M, Edelstein DR. (1989) The large vestibular aqueduct syndrome in children. Arch Otolaryngol Head Neck Surg 115:54–58. Madden C, Halsted M, Benton C, Greinwald J, Choo D. (2003) Enlarged vestibular aqueduct syndrome in the pediatric population. Otol Neurotol 24:625–632. Molini E, Serafini G, Altissimi G, Simoncelli C, Ricci G. (1998) Sudden idiopathic hearing loss: case reports in the course of 10 years. Acta Otorhinolaryngol Ital 18(4):218–227. Mondini C. (1791) Anatomica surdi nati section: bononiensi scientarium et atrium instituto atque academia commentarii. Bonaniae 7:419–428. Nakashima T, Ueda H, Furushashi A, Sato E, Asahi K, Naganawa S, Beppo R. (2000) Air-bone gap and resonant frequency in large vestibular aqueduct syndrome. Am J Otol 21:671–674. Okumura T, Takahashi H, Honjo I, Takagi A, Mitamura K. (1995) Sensorineural hearing loss in patients with large vestibular aqueduct. Laryngoscope 105(3):289–294. Pyle GM. (2000) Embryological development and large vestibular aqueduct syndrome. Laryngoscope 110(11):1837–1842. Shirazi A, Fenton JE, Pagan PA. (1994) Large vestibular aqueduct syndrome and stapes fixation. J Laryngol Otol 108:989–990. Temple RH, Ramsden RT, Axon PR, Saeed SR. (1999) The large vestibular aqueduct syndrome: the role of cochlear implantation in its management. Clin Otolaryngol 24:301–306. Valvasorri GE. (1983) The large vestibular aqueduct and associated anomalies of the inner ear. Otolaryngol Clin North Am 16:95–101. Valvassori GE, Clemis JD. (1978) The large vestibular aqueduct syndrome. Laryngoscope 88(5):723–728. 828