* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download molecular evidence for the common origin of snap
Survey
Document related concepts
History of herbalism wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Plant physiology wikipedia , lookup
Plant breeding wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Ornamental bulbous plant wikipedia , lookup
Plant morphology wikipedia , lookup
Plant ecology wikipedia , lookup
History of botany wikipedia , lookup
Plant reproduction wikipedia , lookup
Flowering plant wikipedia , lookup
Plant evolutionary developmental biology wikipedia , lookup
Glossary of plant morphology wikipedia , lookup
Perovskia atriplicifolia wikipedia , lookup
Transcript
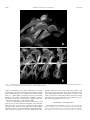
American Journal of Botany 89(9): 1503–1509. 2002. MOLECULAR EVIDENCE FOR THE COMMON ORIGIN SNAP-TRAPS AMONG CARNIVOROUS PLANTS1 KENNETH M. CAMERON,2,4 KENNETH J. WURDACK,2,5 RICHARD W. JOBSON2,3 OF AND The Lewis B. and Dorothy Cullman Program for Molecular Systematics Studies, The New York Botanical Garden, Bronx, New York 10458 USA; and 3 Department of Botany, University of Queensland, Brisbane, Queensland 4072 Australia 2 The snap-trap leaves of the aquatic waterwheel plant (Aldrovanda) resemble those of Venus’ flytrap (Dionaea), its distribution and habit are reminiscent of bladderworts (Utricularia), but it shares many reproductive characters with sundews (Drosera). Moreover, Aldrovanda has never been included in molecular phylogenetic studies, so it has been unclear whether snap-traps evolved only once or more than once among angiosperms. Using sequences from nuclear 18S and plastid rbcL, atpB, and matK genes, we show that Aldrovanda is sister to Dionaea, and this pair is sister to Drosera. Our results indicate that snap-traps are derived from flypaper-traps and have a common ancestry among flowering plants, despite the fact that this mechanism is used by both a terrestrial species and an aquatic one. Genetic and fossil evidence for the close relationship between these unique and threatened organisms indicate that carnivory evolved from a common ancestor within this caryophyllid clade at least 65 million years ago. Key words: Aldrovanda; carnivorous plants; Dionaea; DNA; Droseraceae; molecular systematics; phylogeny. Charles Darwin (1875) called Dionaea muscipula Ellis (Venus’ flytrap) ‘‘one of the most wonderful plants in the world’’ because of its rapidly closing, snap-trap leaves, which are used to capture and digest small animals for the plants’ nutritional benefit. Its trapping mechanism is one of the fastest movements known among plants (Iijima and Sibaoka, 1985; Juniper, Robins, and Joel, 1989), and the monotypic genus is well known to both schoolchildren and scientists. Few people, however, are as familiar with the aquatic waterwheel plant (Aldrovanda vesiculosa L.), which employs a similar snap-trap mechanism for trapping prey underwater (Fig. 1). Aldrovanda vesiculosa was first collected from India in 1696 (Kundu, Basu, and Chakraverty, 1996). The genus was named in honor of Ulisse Aldrovandi, a prominent Italian naturalist, and the specific epithet used by Linnaeus in 1753 refers to the fact that the leaves were originally thought to trap air for buoyancy (de Lassus, 1861; Lloyd, 1976). Darwin (1875) carefully studied living specimens and suspected the plant, instead, to use its leaves for small animal capture, as was proven later to be the case. Aldrovanda is a rootless, submerged, aquatic herb that produces whorls of 7–8 leaves per node. It grows in dystrophic lakes and ponds throughout the Old World. In its habit and distribution, therefore, it is similar to many aquatic species of the carnivorous bladderworts (Utricularia spp.). Rather than producing underwater bladders for catching prey, however, the leaves of Aldrovanda consist of a pair of oval or round leaf lobes, 5–10 mm long, that resemble an open bivalve shell. On the inner surface of each lobe are approximately 20 hairs that serve as triggers for the snap-trap. This foliar morphology is so strongly reminiscent of the leaves Manuscript received 26 February 2002; revision accepted 2 May 2002. The authors thank Bruce Salmon for sending us leaf tissue of Drosera regia and Jay Horn at Duke University for material of Dionaea and Drosophyllum. The photograph of Aldrovanda was given with permission by Mr. Barry Rice, qwww.sarracenia.com. This research was funded by the Lewis B. and Dorothy Cullman Foundation. 4 Author for reprint requests (e-mail: [email protected]). 5 Current address: Laboratory of Molecular Systematics, Museum Support Center A2000, Smithsonian Institution, Washington, D.C. 20560 USA. 1 of Venus’ flytrap that Darwin (1875) described Aldrovanda as ‘‘a miniature, aquatic Dionaea.’’ In its reproductive morphology, however, Aldrovanda shares more characters with the carnivorous sundews (Drosera spp.) than it does with Dionaea. A comparison of these taxa is presented in Table 1. Both Aldrovanda and Drosera have flowers with five stamens and parietal placentation of their ovules, whereas the flowers of Dionaea have 15 stamens and basal placentation. The pistil of Aldrovanda has separate, undivided styles like some species of Drosera, whereas the styles of Dionaea are completely united. Both Dionaea and Drosera display multiple operculate pores around the connection area of their pollen tetrads (Takahashi and Sohma, 1982). Aldrovanda also sheds its pollen as tetrads, but its pollen is triporate and differs from both Dionaea and Drosera. Consequently, Nakai (1949) proposed a monotypic family, Aldrovandaceae, to accommodate this enigmatic taxon rather than to classify it in Droseraceae. A close relationship with Drosera is further supported by genetic evidence in the form of chromosome size, centromere position, and CMA1 DAPI2 fluorescent banding patterns (Hoshi and Kondo, 1998). Both Aldrovanda and Drosera have mostly small chromosomes with no primary constriction and 2–4 CMA1 DAPI2 sites. In contrast, the chromosomes of Dionaea are all medium in size with localized centromeres and show no CMA1 DAPI2 sites. The only cladistic study of phylogenetic relationships within Droseraceae that sampled Aldrovanda included 14 morphological characters for this taxon (Williams, Albert, and Chase, 1994). The results showed a sister relationship between Aldrovanda and Drosera, with Dionaea sister to the pair, implying that plants with adhesive, glandular flypaper-traps may have evolved from plants with snap-traps. This scenario seems unlikely because there is evidence (Williams, 1976; Juniper, Robins, and Joel, 1989) that the trigger hairs of Dionaea are derived from glandular trichomes, not vice versa. On the other hand, a number of molecular phylogenetic studies have demonstrated that carnivory evolved independently among several different lineages of flowering plants (Albert, Williams, and 1503 1504 AMERICAN JOURNAL OF BOTANY [Vol. 89 Fig. 1. Habit photographs of Dionaea muscipula Ellis and Aldrovanda vesiculosa L., which capture and digest small animals with modified snap-trap leaves. (a) Dionaea is a terrestrial species. (b) Aldrovanda is a rootless, submerged aquatic. Chase, 1992; Meimberg et al., 2000). Furthermore, convergent evolution has selected for similar trapping mechanisms among these unrelated lineages. For example, leaves used as pitfalltraps (i.e., ‘‘pitcher plants’’) are known to have evolved within lineages of the orders Caryophyllales, Oxalidales, Ericales, and Poales (sensu APG, 1998). Likewise, flypaper-traps have evolved in at least three or four different clades. The exact relationship between Aldrovanda, Dionaea, and Drosera has not been addressed adequately, and molecular data clearly have a role to play in sorting out this problem (Givnish, 1989). With full knowledge that similar trapping mechanisms have evolved independently among different lineages of carnivorous plants, we set out to determine the phy- logenetic position of Aldrovanda using DNA sequence data from both nuclear and plastid genes. The placement of this taxon among angiosperms has implications not only for taxonomy, but also for determining whether snap-traps evolved more than once among angiosperms and whether they were derived from flypaper-traps or vice versa. MATERIALS AND METHODS Taxon sampling and gene sequencing—Partial rbcL, atpB, and matK plastid gene sequences, as well as nuclear small-subunit (18S) ribosomal DNA sequences were obtained from 15 taxa classified in the order Caryophyllales. DNA sequences for 18S and atpB were newly generated for Aldrovanda, September 2002] TABLE 1. CAMERON ET AL.—ORIGIN OF SNAP-TRAPS 1505 A comparison of nonmolecular characters between Aldrovanda and other members of Droseraceae s.s. Character Distribution Habit Trapping mechanism Aldrovanda Old World: Disjunct in Europe, Africa, Southeast Asia, and Australia Aquatic Snap-trap with ;20 trigger hairs/leaf lobe Circinate Sessile, nonvascular, quadrifid adaxial glands Dionaea Drosera regia Other Drosera spp. New World: Southeast USA Old World: South Africa Terrestrial Flypaper-trap New and Old World: esp. diverse in South Africa and Australia Terrestrial Flypaper-trap Circinate Stalked, vascular, adaxial glands Circinate Stalked, vascular, adaxial glands Stamen no. Pollen 5 Triaperturate, spinose pollen shed in tetrads without radial plates Terrestrial Snap-trap with 3 trigger hairs/ leaf lobe Circinate Sessile, nonvascular, adaxial glands and stellate, abaxial glands 15 Multi-aperturate, nonspinose pollen shed in tetrads without radial plates Pollen apertures Pores opposite at tetrad connection; not operculate and without channel openings Pores alternate at tetrad connection; not operculate and without channel openings Styles Placentation Seed Separate, undivided Parietal Large, with smooth, thickened exotesta and endotesta United Basal Large, with smooth, thickened, exotesta Chromosome no. 2n 5 48 (medium) 2n 5 32 (small) 2.94 mm2 2 0.90 mm2 None ? ? 0.51–1.69 mm2 Mostly 2 or more 2/1 2/1 1/1 2 or 1/2 or 1 Leaf vernation Glands Average area of chromosome complement CMA1 DAPI2 sites 7-methyljuglone/plumbagin Dionaea, Drosophyllum, Drosera regia, D. adelae, and D. capensis. We also generated new rbcL sequences for Aldrovanda and Drosera adelae, as well as new matK sequences for Aldrovanda, D. capensis, and D. adelae. All remaining sequences used in the analyses were downloaded from GenBank (See http://ajbsupp.botany.org/v89/ for voucher information and GenBank accession numbers). We initially determined (results not shown) that Aldrovanda is a member of the ‘‘non-core’’ Caryophyllales (sensu Cuénoud et al., 2002) by analyzing its rbcL, atpB, and 18S sequences within a large, published matrix of angiosperm sequences that included representatives of most eudicot orders (modified from Soltis et al., 2000). Based on this result and on previously published, broadscale analyses of Caryophyllales (Lledó et al., 1998; Meimberg et al., 2000; Soltis et al., 2000; Cuénoud et al., 2002), we sampled 15 species from 13 genera and nine families (Ancistrocladaceae, Dioncophyllaceae, Droseraceae, Frankeniaceae, Nepenthaceae, Plumbaginaceae, Polygonaceae, Simmondsiaceae, and Tamaricaceae) to represent the ingroup and appropriate outgroup taxa. The entire aligned matrix is available from the first author or online from The New York Botanical Garden (http://www.nybg.org). Total DNA was extracted using the FastPrep (Qbiogene, Carlsbad, California, USA) and glassmilk method from approximately 0.5 cm2 of dried tissue as described by Struwe et al. (1998). Target loci were amplified in 50-mL volumes using standard polymerase chain reaction (PCR) protocols that included the addition of bovine serum albumin (BSA) and betaine. Amplification and sequencing primers were the same as used by Soltis et al. (2000) except for matK, which we amplified and sequenced using primers 390F and 1326R as cited in Cuénoud et al. (2002). This resulted in ;900 base pairs (bp) of sequence for that gene. In all cases, resulting PCR products were purified using QIAquickTM spin columns (Qiagen, Valencia, California, USA) according to the manufacturer’s protocols. Cycle sequencing reactions were completed using a combination of purified PCR template, primer, and dRhodamine Ready Reaction mix (Applied Biosystems, Foster City, California, USA) for 20 cycles. These reactions resulted in nearly complete, overlapping, forward and reverse strands of the target loci. Centri-Sep sephadex columns (Princeton Separations, Adelphia, New Jersey, USA) were used according to 5 5 Multi-aperturate, spinose Multi-aperturate, spinupollen shed in tetrads lose pollen shed in without radial plates tetrads mostly with radial plates Pores opposite at tetrad Pores opposite at tetrad connection; not operconnection; opercuculate and without late and mostly with channel openings channel openings Separate, undivided Separate, mostly divided Parietal Parietal Small, dust-like with Small, dust-like with thin, reticulate exotesthin, reticulate exotesta ta 2n 5 34 (small) 2n 5 20, 30, 32, 40, 60, 80 (small) the manufacturer’s instructions to remove excess dye terminators and primer from the cycle sequencing products. These were subsequently dehydrated, resuspended in a mixture of formamide and loading dye, and pipetted onto a 5% denaturing polyacrylamide gel. Samples were run on an Applied Biosystems ABI 377XL automated DNA sequencer, and resulting electropherograms were edited using Sequencher 3.0 (GeneCode, Ann Arbor, Michigan, USA). Phylogenetic analyses—The individual and combined data matrices were analyzed using the parsimony criterion in PAUP* version 4.0b8 (Swofford, 2001). Simmondsia was specified as the single outgroup taxon based on the topologies produced in broader phylogenetic studies (e.g., Soltis et al., 2000; Cuénoud et al., 2002). The 59 and 39 ends of each gene sequence (typically ;30–50 bp) were excluded to minimize missing data. In addition, a hypervariable 32-bp region of matK located at ;190 bp from the 59 end was excluded because of ambiguous alignment. All equally parsimonious trees were found by executing a branch and bound search with gaps treated as missing data and characters unordered and weighted equally. Internal support was determined by performing parsimony bootstrap analyses (1000 replicates, simple addition, and tree bisection-reconnection [TBR] branch swapping). Although the phylogenetic position of Drosera regia Stephens was found to be inconsistent among the individual gene analyses (i.e., either sister to the other species of Drosera or sister to the Aldrovanda-Dionaea clade), no bootstrap support .50% (i.e., ‘‘hard’’ incongruence sensu Weins, 1998) was detected among the individual trees, so combination of the four data sets proceeded. To address the evolution of trapping mechanisms, we used both ACCTRAN and DELTRAN optimizations in MacClade version 3.04 (Maddison and Maddison, 1992) to examine the distribution of the following character states: not carnivorous, pitfall-trap, flypaper-trap, and snap-trap. RESULTS Table 2 summarizes each individual gene matrix as well as the combined four-gene matrix. Tree statistics for each analysis 1506 TABLE 2. AMERICAN JOURNAL OF BOTANY [Vol. 89 Comparison of matrix and tree statistics. No. characters No. variable characters (% of total) No. informative characters (% of variable) No. trees Tree length (steps) Consistency index (CI) Retention index (RI) Droseraceae monophyly bootstrap support (%) Drosera monophyly bootstrap support (%) Aldrovanda/Dionaea monophyly bootstrap support (%) are also presented. Droseraceae sensu stricto (s.s.) were found to be monophyletic and with 100% bootstrap support in all individual gene analyses. A sister relationship between Aldrovanda and Dionaea was discovered in every analysis, but it is supported by the bootstrap only in the matK analysis. However, an alternative topology in which Aldrovanda alone is sister to Drosera was found to be equally parsimonious in the results of the separate 18S and atpB analyses. In these two cases, the strict consenus trees are unresolved for the position of these taxa (see Fig. 2). The combined data matrix consists of 5386 characters, of which 693 are potentially parsimony informative. We recovered a single most parsimonious tree (Fig. 3) in which every clade is supported at .50% by the bootstrap (bts). The genus Drosera is monophyletic, with the morphologically unique, South African species D. regia sister to the other two species sampled. Dionaea and Aldrovanda are sister to each other (90% bts), and together this pair is sister to Drosera, forming a well-supported Droseraceae (100% bts) if Drosophyllum lu- Nuclear 18S Plastid rbcL Plastid atpB Plastid matK Combined 1739 183 (10.5) 99 (54.1) 5 317 0.700 0.639 100 69 ,50 1331 327 (24.6) 159 (48.6) 3 603 0.697 0.544 100 ,50 ,50 1428 368 (25.8) 184 (50.0) 6 599 0.748 0.683 100 59 ,50 888 439 (49.4) 188 (42.8) 1 858 0.724 0.629 100 ,50 57 5386 1317 (24.5) 693 (52.6) 1 2404 0.712 0.611 100 57 90 sitanicum (L.) Link is excluded from the family. Sister to Droseraceae s.s. is a second clade (81% bts) composed of four genera, all but one of which are carnivores and/or lianas at some stage in their life cycle. These include the carnivorous liana Triphyophyllum, which is sister to the noncarnivorous liana Ancistrocladus (100% bts). The monotypic carnivore Drosophyllum is sister to them (99% bts), and the carnivorous pitcher-plant liana Nepenthes is sister to these three. DISCUSSION Despite Aldrovanda’s similarity in leaf morphology to Dionaea, the greater commonality in floral features between Aldrovanda and Drosera has consistently kept Aldrovanda classified within Droseraceae by most taxonomists (e.g., Diels, 1906), even when Dionaea is elevated to familial status as Dionaeaceae (Small, 1933). In fact, the illegitimate combination Drosera aldrovanda F. Muell. has even been made (von Mueller, 1876) to accommodate Aldrovanda within Drosera Fig. 2. Alternative topologies for the relationships within Droseraceae based on individual gene analyses and on the analysis of morphological data by Williams, Albert, and Chase (1994). Only the Droseraceae s.s. clade is shown for each as a strict consensus tree with bootstrap percentages. September 2002] CAMERON ET AL.—ORIGIN OF SNAP-TRAPS 1507 Fig. 3. Phylogenetic relationships for the carnivorous plant genera in Caryophyllales inferred from parsimony analysis of combined plastid rbcL, atpB, matK, and nuclear small-subunit (18S) rDNA sequences. Branch lengths are proportional to the number of character changes on each branch as indicated (ACCTRAN optimization). Bootstrap percentages .50% are indicated at each node of the single most parsimonious tree. The following character states were optimized onto the gene tree: not carnivorous (dark gray), pitfall-traps (dashes), flypaper-traps (light gray), and snap-traps (solid black). rather than to treat it as a distinct genus. In contrast, Dionaea has never been classified under any other generic name. The common origin and evolution of snap-traps—Until now, no molecular data have been available to help settle the ongoing debate regarding the exact phylogenetic position and classification of Aldrovanda vesiculosa. However, our results show that Aldrovanda is sister to Dionaea, not to Drosera, and that snap-traps evolved only once among carnivorous plants (moving Aldrovanda away from Dionaea to a position sister to Drosera requires an additional ten steps). We have pointed out the numerous morphological differences between Aldrovanda and Dionaea, but there are other likely synapomorphies in addition to the common trapping mechanism that unite them. Boesewinkel (1989) considered the ovules and seeds of Aldrovanda to be more similar to those of Dionaea than to any other taxon in Droseraceae. The two genera also share a similar trichome/gland morphology, including sessile and stellate glands lacking vascular tissue and multicellular trigger hairs (Juniper, Robins, and Joel, 1989). Moreover, the 1508 AMERICAN JOURNAL traps of both Aldrovanda and Dionaea function in a similar manner by rapid transmission (6–17 cm/s) of action potentials between excitable cells (Iijima and Sibaoka, 1985). Some species of Drosera also generate action potentials, but their trasmission velocity is 10% slower. Finally, both Aldrovanda and Dionaea lack 7-methyljuglone, but possess plumbagin in their tissues (Culham and Gornall, 1994). However, there are several species of Drosera that have this same napthoquinone profile. Based on a number of shared morphological characters and the structure of the molecular cladogram, therefore, we recommend the recognition of Aldrovanda, Dionaea, and Drosera, but not Drosophyllum, as members of Droseraceae s.s. Drosophyllum is a monotypic genus from Portugal. Although it traps insects with glandular trichomes, it differs from Drosera in a number of morphological features, and its disassociation from Droseraceae s.s. has been documented and discussed in detail by others (Williams, Albert, and Chase, 1994; Meimberg et al., 2000). It should be treated as the sole member of Drosophyllaceae. Furthermore, based on this cladogram, it is most parsimonious to hypothesize that the snap-traps of Aldrovanda and Dionaea were derived from a common terrestrial ancestor that had flypaper-traps, not the other way around. Likewise, the pitfall-traps of the tropical pitcher plant genus Nepenthes also must have evolved from an ancestor with adhesive, flypaper traps. Its exact position among the genera of Caryophyllales has been ambiguous to date; Albert, Williams, and Chase, (1992) and Fay et al. (1997) found Nepenthes to be sister to a Plumbago/Rheum clade, Williams, Albert, and Chase (1994) and Lledó et al. (1998) found it sister to the entire carnivorous clade, Meimberg et al. (2000) to the Drosophyllum/Ancistrocladus/Dioncophyllaceae clade, and Cuénoud et al. (2002) to Droseraceae. Only one of these relationships—that of Meimberg et al. (2000), which is the same as found here—has been supported by the bootstrap. Lledó et al. (1998) and others have discussed the hypothesis that the common ancestor of this mostly insectivorous, caryophyllid clade was preadapted for a carnivorous lifestyle because many of the noncarnivorous, outgroup taxa (e.g., Plumbago, Tamarix, Frankenia) possess multicellular glands that secrete mucilage, salt, or other compounds. Modification of these glands for animal capture and/or digestion followed by their co-option as snap-trap trigger hairs in the ancestor of Dionaea and Aldrovanda seems plausible and has been suggested (Juniper, Robins, and Joel, 1989; Williams, 1976). The habitat of Dionaea muscipula frequently floods (Roberts and Oosting, 1958). Yet even under these conditions, it has been reported that the flytraps continue to grow and capture prey underwater (Schnell, 1976). Thus, it is not difficult to envision a Dionaea-like, terrestrial ancestor of Aldrovanda vesiculosa becoming adapted to a permanently aquatic lifestyle as suggested by Arber (1920). Implications for biogeography and conservation—Aldrovanda has a fossil pollen and seed history dating back to the lower Tertiary (von Kircheimer, 1941; Muller, 1981; Yakubovskaya, 1991), with at least 13 different names having been applied to these fossils (e.g., A. siberica V. Nikit., A. europaea Negru, and A. praevesiculosa Kirchh.). Dionaea also has a documented fossil history, with the discovery of pollen from the middle Miocene and Pliocene of central Europe that is comparable to modern Dionaea muscipula (Muller, 1981). OF BOTANY [Vol. 89 These microfossils indicate that both genera were once more common and widespread in distribution than they are today. The ancient seeds of Aldrovanda also show enough morphological variation for Yakubovskaya (1991) to have hypothesized an evolutionary continuum along at least two ancestral lines. One of these, originating from A. ovata M. Chandl., has become extinct; the other, from A. intermediata E. Reid and M. Chandl., ultimately led to the evolution of the modern species. Moreover, these fossils strongly support a date of origin for this entire, highly specialized, carnivorous lineage of at least 65 million years ago and probably much earlier. This age may assist in explaining the current transcontinental distributions and putative Gondwanan origins of Drosera (Meimberg et al., 2000) and Nepenthes (Meimberg et al., 2001). Modern Aldrovanda vesiculosa is a relictual species historically distributed in parts of western Europe, central Africa, southern India, Japan, and Queensland, Australia. It is now presumed to be extinct in several countries (e.g., France, Italy, and India), and is shrinking drastically in the number and size of its extant populations (Adamec, 1995; Kaminski, Adamec, and Breckpot, 1996; Kundu, Basu, and Chakraverty, 1996). For 200 yr, it had been recorded (Adamec and Lev, 1999) from more than 150 sites within Europe. Today it is estimated to survive in fewer than 36 localities, most of which are in Poland, Ukraine, and Russia (Adamec, 1995). For this reason, it is now considered one of the rarest aquatic plants in the Old World (Kaminski, Adamec, and Breckpot, 1996). Likewise, Dionaea muscipula can be considered a relictual species with a narrow, endemic distribution of less than 300 km2 in the southeastern United States. Both Aldrovanda and Dionaea are severely threatened by anthropogenic disturbance of their specialized habitats (Adamec, 1995; Kaminski, Adamec, and Breckpot, 1996; Kundu, Basu, and Chakraverty, 1996), and Dionaea has been overcollected as a horticultural curiosity; it is currently listed on Appendix II of CITES. Recent reintroductions of Aldrovanda into the Czech Republic (Adamec and Lev, 1999) appear to be successful and offer hope of conserving this remarkable species. Introductions of Dionaea have also taken place in Florida, New Jersey, and Deleware (USDA NRCS, 2001), but the future survival of these two species in their native habitats is of serious concern to conservationists. It is our hope that the fundamental systematic data presented here have helped to clarify the classification, phylogenetic relationships, evolutionary origin, and ecological adaptation of what Arthur Dobbs (Dillwyn, 1843), the former Governor of North Carolina and discoverer of Venus’ flytrap, called ‘‘the great wonder of the vegetable kingdom.’’ LITERATURE CITED ADAMEC, L. 1995. Ecological requirements and recent European distribution of the aquatic carnivorous plant Aldrovanda vesiculosa L.: a review. Folia Geobotanica and Phytotaxonomica 30: 53–61. ADAMEC, L., AND J. LEV. 1999. The introduction of the aquatic carnivorous plant Aldrovanda vesiculosa to new potential sites in the Czech Republic: a five-year investigation. Folia Geobotanica and Phytotaxonomica 34: 299–305. ALBERT, V. A., S. E. WILLIAMS, AND M. W. CHASE. 1992. Carnivorous plants: phylogeny and structural evolution. Science 257: 1491–1495. APG [ANGIOSPERM PHYLOGENY GROUP.]. 1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531–553. ARBER, A. 1920. Water plants; a study of aquatic angiosperms. Cambridge University Press, Cambridge, UK. September 2002] CAMERON ET AL.—ORIGIN OF SNAP-TRAPS BOESEWINKEL, F. D. 1989. Ovule and seed development in Droseraceae. Acta Botanica Neerlandica 38: 295–312. CUÉNOUD, P., V. SAVOLAINEN, L. W. CHATROU, M. POWELL, R. J. GRAYER, AND M. W. CHASE. 2002. Molecular phylogenetics of Caryophyllales based on nuclear 18S rDNA and plastid rbcL, atpB, and matK DNA sequences. American Journal of Botany 89: 132–144. CULHAM, A., AND R. J. GORNALL. 1994. The taxonomic significance of naphthoquinones in the Droseraceae. Biochemical Systematics and Ecology 22: 507–515. DARWIN, C. 1875. Insectivorous plants. John Murray, London, UK. DE L ASSUS , A. 1861. Analyse du mémorie de Gaëtan Monti suyr l’Aldrovandia suivie de quelques observations sur l’irritabilité des follicules de cette plante. Bulletin de la Société Botanique de France 8: 519– 523. DIELS, L. 1906. Droseraceae. In A. Engler [ed.], Das Pflanzenreich 4: 1–136. W. Engelmann, Weinheim, Germany. DILLWYN, L. W. 1843. Hortus Collinsonianus; an account of the plants cultivated by the late Peter Collinson, Esq., F.R.S., arranged alphabetically. Imprint published by W. Swansea, C. Murray and D. Rees. FAY, M., K. M. CAMERON, G. PRANCE, M. D. LLEDÓ, AND M. W. CHASE. 1997. Familial relationships of Rhabdodendron (Rhabodendraceae): plastid rbcL sequences indicate a caryophyllid placement. Kew Bulletin 52: 923–932. GIVNISH, T. J. 1989. Ecology and evolution of carnivorous plants. In W. G. Abrahamson [ed.], Plant–animal interactions, 243–290. McGraw-Hill, New York, New York, USA. HOSHI, Y., AND K. KONDO. 1998. A chromosome phylogeny of the Droseraceae by using CMA-DAPI fluorescent banding. Cytologia 63: 329–339. IIJIMA, T., AND T. SIBAOKA. 1985. Membrane potentials in excitable cells of Aldrovanda vesiculosa trap-lobes. Plant and Cell Physiology 26: 1–14. JUNIPER, B. E., R. J. ROBINS, AND D. M. JOEL. 1989. The carnivorous plants. Academic Press, London, UK. KAMINSKI, R., L. ADAMEC, AND C. BRECKPOT. 1996. Report on recent sites of Aldrovanda vesiculosa (Droseraceae) in Poland. Fragmenta Floristica et Geobotanica 41: 291–294. KUNDU, S. R., S. BASU, AND R. K. CHAKRAVERTY. 1996. Aldrovanda vesiculosa Linn.—its maiden appearance and disappearance from India: a review. Journal of Economic and Taxonomic Botany 20: 719–724. LLEDÓ, M., M. CRESPO, K. CAMERON, M. FAY, AND M. CHASE. 1998. Systematics of Plumbaginaceae based upon cladistic analysis of rbcL sequence data. Systematic Botany 23: 21–29. LLOYD, F. E. 1976. The carnivorous plants. Dover, New York, New York, USA. MADDISON, W. P., AND D. R. MADDISON. 1992. MacClade: analysis of phylogeny and characer evolution. Sinauer, Sunderland, Massachusetts, USA. MEIMBERG, H., P. DITTRICH, G. BRINGMANN, J. SCHLAUER, AND G. HEUBL. 1509 2000. Molecular phylogeny of Caryophyllidae s.l. based on matK sequences with special emphasis on carnivorous taxa. Plant Biology 2: 218–228. MEIMBERG, H., A. WISTUBA, P. DITTRICH, AND G. HEUBL. 2001. Molecular phylogeny of Nepenthaceae based on cladistic analysis of plastid trnK intron sequence data. Plant Biology 3: 164–175. MULLER, J. 1981. Fossil pollen records of extant angiosperms. Botanical Review 47: 1–142. NAKAI, T. 1949. Classes, Ordines, Familiae, Subfamiliae, Tribus, Genera nov quae attinet ad plantas Koreanas. Journal of Japanese Botany 24: 8–14. ROBERTS, P. R., AND H. J. OOSTING. 1958. Reponses of Venus’ flytrap to factors involved in its endemism. Ecological Monographs 28: 193–218. SCHNELL, D. E. 1976. Carnivorous plants of the United States and Canada. John F. Blair, Winston-Salem, North Carolina, USA. SMALL, J. K. 1933. Flora of the southeastern United States. Published by the author, New York, New York, USA. SOLTIS, D. E., ET AL. 2000. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society 133: 381–461. STRUWE, L., M. THIV, J. W. KADEREIT, A. S. R. PEPPER, T. J. MOTLEY, P. J. WHITE, J. H. E. ROVA, K. POTGIETER, AND V. A. ALBERT. 1998. Saccifolium (Saccifoliaceae), an endemic of Sierra de la Neblina on the Brazilian-Venezuelan border, is related to a temperate-alpine linage of Gentianaceae. Harvard Papers in Botany 3: 199–214. SWOFFORD, D. L. 2001. PAUP* 4.0b8: phylogenetic analysis using parsimony. Sinauer, Sunderland, Massachusetts, USA. TAKAHASHI, H., AND K. SOHMA. 1982. Pollen morphology of the Droseraceae and its related taxa. Science Reports, Tohoku University, 4th Series, Biology 38: 81–156. USDA NRCS. 2001. The PLANTS database, version 3.1 (http:// plants.usda.gov). National Plant Data Center, Baton Rouge, Louisiana, USA. VON KIRCHEIMER, F. 1941. Über ein Vorkommen der Gattung Aldrovanda Linné im Alttertiar Thüringens. Braunkohle 40: 308–311. VON MUELLER, F. J. H. 1876. Fragmenta Phytographiae Australiae, vol. 10: 79–81. Published by the author, Melbourne, Australia. WEINS, J. J. 1998. Combining data sets with different phylogenetic histories. Systematic Biology 47: 568–581. WILLIAMS, S. E. 1976. Comparative sensory physiology of the Droseraceae— the evolution of a plant sensory system. Proceedings of the American Philosophical Society 120: 187–204. WILLIAMS, S. E., V. A. ALBERT, AND M. W. CHASE. 1994. Relationships of Droseraceae: a cladistic analysis of rbcL sequence and morphological data. Ameerican Journal of Botany 81: 1027–1037. YAKUBOVSKAYA, T. V. 1991. The genus Aldrovanda (Droseraceae) in the Pleistocene of the Belorussian SSR. Botanicheskii Zhurnal (St. Petersburg) 76: 109–118.