* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sterilant
Survey
Document related concepts
Transcript
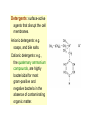
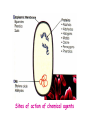
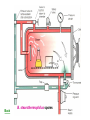
Disinfection and Sterilization Disinfection: killing of most microbial forms. Disinfectant: a chemical substance used to kill microbes on surfaces but too toxic to be applied directly to tissue. Antisepsis: inhibit or eliminate microbes on skin or other living tissue. Sterilization: removal of life of every kind by physical or chemical methods. Sterilant: an agent or method used to remove or kill all microbes. Septic: presence of pathogenic microbes in living tissue. Aseptic: absence of pathogenic microbes. Sterile: free of life of every kind. Bacteriostatic: inhibiting bacterial multiplication. Bacteriostatic action is reversible by removal or inactivation of agent. Bactericidal: killing bacteria. Modes of action of antimicrobial agents 1. Disruption of cell membrane or wall 2. Damage to DNA Formation of pyrimidine dimer by UV irradiation Single- or double-strand DNA break by ionizing radiation DNA reactive chemicals, e.g. alkylating agents 3. Protein denaturation 4. Removal of free sulfhydryl groups Formation of disulfide bond by oxidizing agents Heavy metals combined with sulfhydryls 5. Chemical antagonism: interference with the normal reaction between an enzyme and its substrate. Methods of sterilization (I) Methods of sterilization (II) Methods of disinfection Effectiveness of disinfectants is influenced by 1. nature of the item, 2. number and resilience of the contaminants, 3. amount of organic material present, 4. type and concentration of disinfectant, and 5. duration and temperature of exposure. High-level disinfectants Used for items involved in invasive procedures. Intermediate-level disinfectants Used for cleaning surface or instruments without bacterial spores and highly resilient organism Low-level disinfectants Used to treat non-critical instruments and devices Antiseptic agents Reduce the number of microbes on skin Triclosan (chlorinated aromatic), chlorhexidine, parachlorometaxylenol (PCMX), and hexachlorophene are commonly used for body or hand cleansing. Physical methods (moist heat, dry heat, filtration, radiation) Heat (moist heat; dry heat) The most common sterilizing methods used in hospitals, suitable for most materials except those that are heatsensitive or consist of toxic or volatile chemicals. Dry heat Dry oven: 160 oC for 2 hrs or 171 oC for 1 hr. (use B. subtilis spores to monitor the effectiveness) Flaming; incineration Moist heat Boiling: boiling for 10 min. is sufficient for killing most vegetative forms of bacteria. Killing of spores requires much longer time. Addition of 2% Na2CO2 or 0.1% NaOH may enhance destruction of spores and prevent rusting of the metal wares. Low temperature disinfection (Pasteurization): 6265 oC for 30 min. or 71.5 oC for 15 sec. This is mainly used for disinfection of milk. Autoclave: 121.5 oC for 15-20 min. in an autoclave. Kill both the vegetative and spore forms of the bacteria. Radiation UV-light: UV-radiation causes damage to DNA (requires direct exposure). Ionizing radiation (microwaves or grays). Filtration The pore size for filtering bacteria, yeasts, and fungi is in the range of 0.22-0.45 mm (filtration membranes are most popular for this purpose). HEPA (high-efficiency particulate air) filters Chemical methods Alcohols: protein denaturant. 70% aqueous solution of ethanol and isopropanol are commonly used as skin disinfectants. Inactivated by organic matter. Oxidizing agents: inactivate cells by oxidizing free sulfhydryl group, e.g., ozone, peracetic acid, iodine, iodophore (similar to alcohols in range of activity; acts rapidly; frequently used with alcohol for disinfecting the skin), H2O2 (3-6%), H2O2 vapor (30%, 55-60 oC), and chlorine compounds: hypochlorite, and chlorine etc. Plasma gas sterilization: H2O2 vapors treated with microwave or radio energy to produce reactive free radicals; no toxic byproducts. An efficient sterilization for dry surfaces. Phenolics: phenol and phenolic compounds (e.g. lysol) lyse the cell membrane and denature proteins at 12% (aqueous solution). Effective for mycobacteria. Hexachlorophene, Trichlosan, and PCMX are also phenolics. Alkylating agents Formalin (37% aqueous solution of formaldehyde); Glutaraldehyde. Both are sporicidal. Toxic for viable tissues. Inactivated by organic matters. Ethylene oxide gas (made nonexplosive by mixing with CO2 or a fluorocarbon): a reliable disinfectant for dry surfaces. Gas must be dissipated by aeration for 16 h before the item can be used. Detergents: surface-active agents that disrupt the cell membranes. Anionic detergents: e.g. soaps, and bile salts. Cationic detergents: e.g., the quaternary ammonium compounds, are highly bactericidal for most gram-positive and negative bacteria in the absence of contaminating organic matter. Sites of action of chemical agents Back B. stearothermophilus spores