* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Part 1: Elements and light
Survey
Document related concepts
Transcript
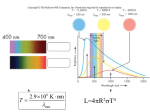
Name: ____________________________ Period: _____ Date: ___________________________ Stellar spectra Inquiry Questions: 1. Why do objects appear the color they are (why is something red, red?) 2. Do objects that have a color give off (not reflect!) all wavelengths of light or just certain ones? Explain. 3. What is a spectrum and what info does it tell you? Part 1: Elements and light Materials: salts flame Procedure: 1. I’m going to put certain salts into a flame and you need to record the appearance of the powder and the color of the flame Data: Salt: Strontium Chloride Formula CuCl2 CuSO4 Sodium Bicarbonate NaHCO3 Sodium Chloride LiCl Barium Chloride Potassium Chloride CoCl2 Calcium Chloride Appearance dry Flame color Conclusions: 1. Did any have the same color? Which ones? 2. Does it seem to be the first part of the chemical formula or the second part that decides the flame color (is it the calcium or the chloride)? Explain. What color would you predict for: 3. Potassium nitrate Potassium sulfate 4. Calcium nitrate Cobalt carbonate 5. How is the colored light produced? This really involves 2 things we studied this year…electrons/energy levels (pg. 107) and star color (pg. 669). 6. Is each element unique in the color it gives off? Explain. Part 2: Stellar spectra Background: We are going to look at some spectra (plural of spectrum) of light that certain elements give off. We can measure them using a spectroscope, which has a diffraction grating, which is a small piece of plastic with slits cut in it. What you are seeing is called a bright line or emission spectrum. It represents the wavelengths of light given off by each element. The opposite (all light with a few dark bands in the same place) is called a dark line or absorption spectrum and represents wavelengths of light absorbed by that element when white light shines through it. Materials: gases diffraction gratings colored pencils Procedure: 1. Look at the tube and draw the spectrum you see. Try to be as accurate as possible about the spacings/numbers. For the description, write the number of lines, colors and numbers. For example, 1 purple line at 7, 2 blue/green ones at 5.8 Data: Light source/element Spectrum: Describe: White light (outside) 7 6 5 4 Light bulb 7 6 5 4 Fluorescent light 7 6 5 4 Hydrogen 7 6 5 4 Helium 7 6 5 4 7 6 5 4 Neon Conclusions: 1. Describe what happens to waves during diffraction. (pg. 473) 2. Why are the spectra from different gases, different? (what is happening to the light to make it look different) 3. Describe how a scientist can tell what elements a star is made of . 4. How are the spectra like fingerprints? 5. What would our star’s spectrum most likely look like? You can draw or describe, but please use numbers for a scale. Part 3: More spectroscopy Procedure: 1. Cut out the 4 unknown stars (A, B, C, D) You can either separate them (but make sure they’re labeled or keep them as one “chunk” and just fold over the ones you’re not using. You can also color the lines, if you wish. 2. Hold each unknown star next to each element on the figure on the next page. Note in Data Table 1 the elements present. 3. Use Data Table 2 to find the stellar class and temperature of each star. Note this in Data Table 1. Strong lines are ones that are thicker. Weak lines are ones that are thinner. 4. Use the HR diagram provided to find the color of each star. Note this in Data Table 1. Data Table 1: Star: Elements present: A B C D Stellar class: Temperature: Color: Data Table 2: Stellar class: O B A F G K M Temperature: > 25,000 K 11,000 - 25,000 K 7,500 - 11,000 K 6,000 – 7,500 K 5,000 – 6,000 K 3,500 – 5,000 K < 3,500 K Elements present: Helium and weak hydrogen lines Helium and strong hydrogen lines Very strong hydrogen lines, others present Weak hydrogen lines, others present, esp. calcium and iron Similar to F, weaker lines, stronger calcium Metals (like iron, mercury) some molecules No elements, strong molecules Conclusions: 1. Can you tell the type of star (dwarf, main sequence or giant) from the stellar class? 2. Look at your HR diagram. What stellar class is the Sun? 3. The sun is class… because… Describe what the spectrum would look like for a 4. Blue Giant 5. Main sequence blue star 6. Main sequence orange star 7. Red Giant 8. White dwarf Giant stars get hot enough to make metals in their cores, while main sequence do not get that hot. 9. How can you tell the spectrum of a main sequence red from a red giant? Could they be different classes? Sometimes the lines don’t fit the usual pattern. They are spaced apart the same, but shifted towards one end of the spectrum. 10. What is this shift called (there are 2)? 11. What does it tell you about the star? 12. Why does it shift (what is happening to wavelength and what’s this called)? 13. Compare star B and D. how are they different and what does this mean? Sometimes the lines are in the right place, but are thicker than usual. This may mean it is spinning faster than usual. 14. Compare stars B & C. What do you notice and what does this mean? Cut out the 4 unknown stars (A, B, C, D) below. You can either separate them (but make sure they’re labeled or keep them as one “chunk” and just fold over the ones you’re not using. You can also color the lines, if you wish. Hold each unknown star next to each element on the figure to the left. You may have to move them a little to see the match. Note in Data Table 1 the elements present.