* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download K-feldspar, feldspathoids
Survey
Document related concepts
Transcript
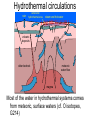
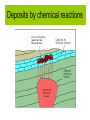
Sub-solidus evolution • Mineral transformations • Secondary minerals • Fluids expulsion and movement – Pegmatite/aplite veins – Mineralized veins • Hydrothermal alteration – Episyenites, endoskarns, greisens – Exoskarns Mineral transformations • Polymorphs • Exsolutions (solvus) Stishovite Pressure (GPa) Phase diagram for SiO2 10 8 6 Coesite 4 2 - quartz - quartz Liquid Cristobalite Tridymite 600 1000 1400 1800 2200 Temperature oC 2600 Feldspar solvus Perthites Opx-Cpx exsolution Secondary minerals • « Autometamorphism » Water-saturated solidus (granites) Secondary minerals • Px => Amp => Bt • Px, Amp, Bt => chlorite (phyllosilicate) • K-feldspar, feldspathoids => sericite (fine white mica) • Ca-plagioclase => saussurite (epidote) • Olivine => serpentine (complex phyllosilicate), iddingsite (a mixture of various Fe-Mg silicates) Figure 3-20. a. Pyroxene largely replaced by hornblende. Some pyroxene remains as light areas (Pyx) in the hornblende core. Width 1 mm. b. Chlorite (green) replaces biotite (dark brown) at the rim and along cleavages. Tonalite. San Diego, CA. Width 0.3 mm. © John Winter and Prentice Hall. Pyx Hbl Chl Bt Sericitization K-feldspar to sericite: 3 KAlSi3O8 + 2 H+ > KAl3Si3O10(OH)2 + 6 SiO2 + 2 K+ Saussuritization Dolerite from ODP leg 180 (sea of Java) Olivine with iddingsite alteration Calcite vein Fluid expulsion • Typical water contents: 2-4% in a granite • Water content of a biotite: ~2 % • Biotite: max. 5-10 % of the rock Excess water = ? + meteoric water also feeding the hydrothermal system Hydrothermal circulations rain sinter and hydrothermal ores steam and hot water o 0 20 older bedrock 300 o volcanic deposits meteoric water flow magma Most of the water in hydrothermal systems comes from meteoric, surface waters (cf. O isotopes, G214) Effect of free, hot water • Overpressure, fractures, etc. • Very aggressive solvent! • Aplite/pegmatite veins Pegmatites recording the same strain pattern as ductile structures Cape de Creus, Spain Quartz solubility in hydrothermal fluids 0.5 mol/kg water = 30 g/l 1 km3 of pluton At 3 wt% H2O = 2.7 1012 kg rock ≈ 1011 kg water Can dissolve 3 109 kg of SiO2, or 106 m3 G.B. Arehart, http://equinox.unr.edu/homepage/arehart/Courses/713/Syllabus.htm Evidence for Si-rich hydrothermal fluids Tatio hydrothermal field, Peru Network of pegmatites/apl ite dykes Mineralized veins • Very incompatible elements (large ions, typically) concentrated in last liquids, then in fluids • The same elements are leached from an already cooled rock (igneous intrusion or its wall-rock) • Precipitate with hydrothermal veins Analysis of hydrothermal fluids from inclusions in pegmatites Gold-quartz veins • See economic geology (GEOL344) pH control on solubility Changes of pH can precipitate ore bodies: •mixing with acid groundwater •Interaction with rocks of very different chemistry (e.g., carbonates, very mafic rocks…) G.B. Arehart, http://equinox.unr.edu/homepage/arehart/Courses/713/Syllabus.htm Barberton gold fields Hydrothermal modifications of rocks • Around the intrusion – Exoskarns, etc. • In the intrusive rocks – Episyenites – Endoskarns, greisens Around the pluton Deposits by chemical reactions Outside the pluton: skarn In the pluton pH control on solubility High pH helps to dissolve SiO2 G.B. Arehart, http://equinox.unr.edu/homepage/arehart/Courses/713/Syllabus.htm In the pluton Loss of quartz => « syenites » (Episyenites) Fedlspar alteration in the pluton • K-feldspar to sericite: 3 KAlSi3O8 + 2 H+ > KAl3Si3O10(OH)2 + 6 SiO2 + 2 K+ • Sericite to kaolin: 2 KAl3Si3O10(OH)2 + 2 H+ + 3 H20 > 3 Al2Si2O5(OH)4 + 2 K+ Requires acidic fluids! In the pluton • Episyenites are plutonic rocks from which the quartz has been dissolved away (therefore, they become syenites) (high pH) • Greisens are plutonic rocks where the feldspar has been transformed into clays (kaolinite) by hydrothermal reactions (low pH)