* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download UNITAID
Health equity wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Maternal health wikipedia , lookup
Epidemiology of HIV/AIDS wikipedia , lookup
Reproductive health wikipedia , lookup
Nutrition transition wikipedia , lookup
Prescription costs wikipedia , lookup
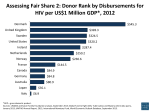
UNITAID An innovative mechanism for scaling up access to medicines and diagnostics for HIV/AIDS, tuberculosis and malaria Jorge Bermudez, Executive Secretary, UNITAID WHO/UNICEF Technical Briefing Seminar on Essential Medicines Policies, 18 November 2009, Geneva Innovative mechanisms: a long way forward Building the way The Leading Group on Innovative Financing (Paris Conference 2006): Sixth plenary session of the Leading Group 2000 - The Millennium Development Goals 2002 - Monterrey International Conference on Financing for Development 2004 - The Global Action against Hunger and Poverty 2005 - New York Declaration on Innovative Sources of Financing for Development 55 member countries and 3 observer countries, major international organizations and NGOs – a platform for discussion and promotion of innovative financing New resources, New sectors, New mechanisms (Proof by example: why expand innovation?). Paris, 28-29 May 2009 A global challenge for Health… Medicines are in the North, patients in the South Less developed countries represent: 84% of the world population Less than 11% of the global health expenditure More than 93% of the disease burden globally UNITAID's role within Global Health System • Aligns with major internationally-agreed goals, such as the Millennium Development Goals • Complements work of other global health actors • Funds and works through Implementing Partners engaged in improving access to health products • Engages with NGOs and civil society to ensure that the needs of patients and communities are met • Ensures partners work meets country demand and procedures and aligns with national health systems Rationale: Why UNITAID?: • Strategically deployed funds in time-limited interventions • Credible funding commitments necessary for sustained market impact • Targeted interventions in global markets to improve patient access to health products • Leverage investments to produce global public goods that generate positive externalities • Ability to transition at the end of the project is essential to ensure sustainability Five founding countries (September 2006) Official signature of the five founding countries when UNITAID was launched, on 19 September 2006, at the United Nations General Assembly, New York UNITAID membership From 5 founding countries (2006): Brazil, Chile, France, Norway, UK Now (2009): supported by 29 countries and the Gates foundation 5 further countries in the final stages of negotiation and to join shortly Resource Mobilization: • Funding from multiple countries from both North and South • Predictable funding gives UNITAID flexibility to respond quickly • UNITAID will pursue measures that increase funding through – Strengthen donor commitments – Increased number of country contributors – Support the Voluntary Solidarity Contributions on airline tickets, through the Millennium Foundation Long term financing = commitment to projects = ability to impact markets A flexible 'air tax' approach Mission, Goals & Objectives: Mission UNITAID’s mission is to contribute to scaling up access to treatment for HIV/AIDS, malaria and tuberculosis, primarily for people in low-income countries, by leveraging price reductions for quality diagnostics and medicines and accelerating the pace at which these are made available. [Constitution] GOAL Using innovative, global market based approaches to improve public health by increasing access to quality products to treat, diagnose and prevent HIV/AIDS, tuberculosis, malaria and related comorbidities in developing countries. Objectives To increase access to efficacious, safe and assured quality products that address Public Health problems To support adaptation of products targeting specific populations To ensure affordable and sustainably priced products To assure availability in sufficient quantities and timely delivery to patients 93 countries already receive UNITAID support… HIV / AIDS 49 recipient countries Malaria 29 recipient countries - Pediatric ARV - Second line ARV -PMTCT - ACT - LLIN - AMFm US$476 m US$318 m - Cross cutting programs: Tuberculosis 72 recipient countries - First line TB Paediatric TB MDR-TB Diagnostics US$211 m US$109 m for PQ of drugs & diagnostics and transversal programs UNITAID funded projects worldwide Tuberculosis Malaria HIV/AIDS 3 0 9 24 4 6 Europe 6 0 2 North Africa and the Middle East 7 0 2 Sub Saharan Africa 32 25 29 Total 72 29 49 Americas Asia Refining current strategy Identify Public Health problems Public health impact?! Additional Complementary Efficient Globally equitable Pro-health approach to intellectual property Transparent Effective Flexible Innovative Forward looking Identify Market shortcomings - Propose interventions to address market shortcomings - Assess the Expected Market impact Deliveries by the end of 2008 QUANTITIES DOLLAR VALUES Number of treatments delivered Update end 2008 - WB income classification LI HIV/AIDS TB 404,443 Malaria 889,398 LMI Dollar amount UMI $ Value Total 64,680 10,817 479,940 HIV/AIDS 91,673 11,277 992,348 TB Malaria 10,049,374 259,276 0 10,308,650 Malaria Total 11,343,215 415,629 22,094 11,780,938 Total 148'968'528 28'559'294 9'865'711 US$ 187'393'532 Better products at lower price Pediatric ARVs Before: Single dose syrups 16 bottles of syrup monthly US$ 200 per patient per year Now (partnering with CHAI): Fixed dose combination 3 tablets a day US$ 60 per patient per year Better products at lower price Tuberculosis • A rotating stockpile that treats 5800 patients a year • New faster diagnostics that can detect MDR-TB in just two days (previous test took six weeks) Better products at lower price Malaria • Investing in prevention with long lasting insecticide treated bed nets • Provision of ACTs at lower costs • UNITAID's recent commitment to the AMFm What is a Patent Pool? What is a Patent Pool? Portfolio of patents and other relevant IP held by various actors made available on a non-exclusive basis to third parties, (e.g. generic manufacturers) against the payment of royalties. Patent Pools come in different shapes and forms and are set up for different purposes While we learn from existing 'standards' pool, the ARV Patent Pool Initiative is quite different History of Medicines Patent Pool 2006 2008 2009 CIPIH 2006 recommendation: "Patent pools of upstream technologies may be useful in some circumstances to promote innovation relevant to developing countries.” May 2008 WHO Global Strategy and Plan of Action included Patent Pools Currently working on a full implementation plan. and proposed to UNITAID to set up a medicines patent pool. July 2008 UNITAID EB ‘Green light 'to establish a medicines patent pool Patents on New ARVs Product +/- Expiry date Atazanavir (Novartis) 2017 Darunavir (Tibotec) 2023 Etravirine (Tibotec) 2019 Fosamprenavir (GSK) 2018 Raltegravir (MSD) 2025 Ritonavir hs (Abbott) 2024 Tenofovir DF (Gilead) 2018 Maraviroc (Pfizer) 2019 21 President Obama on humanitarian licensing • Increase Access to Affordable Drugs: Barack Obama and Joe Biden believe that people in developing countries living with HIV/AIDS should have access to safe, affordable generic drugs to treat HIV/AIDS. They will break the stranglehold that a few big drug and insurance companies have on these life-saving drugs. They support the rights of sovereign nations to access quality-assured, low-cost generic medication to meet their pressing public health needs under the WTO’s Declaration on Trade Related Aspects of Intellectual Property Rights (TRIPS). Barack Obama and Joe Biden also support the adoption of humanitarian licensing policies that ensure medications developed with U.S. taxpayer dollars are available off-patent in developing countries. 22 http://www.barackobama.com/pdf/issues/FactSheetAIDS.pdf Similar thinking in different settings… • “On the part of rich countries, there is excessive zeal for protecting knowledge through an unduly rigid assertion of the right to intellectual property, especially in the field of health care.” – – *“Encyclical Letter Caritas In Veritate Of The Supreme Pontiff Benedict XVI To The Bishops Priests And Deacons Men 23 And And Women Religious The Lay Faithful And All People Of Good Will On Integral Human Development In Charity Truth,” June 29, 2009. http://www.vatican.va/holy_father/benedict_xvi/encyclicals/documents/hf_ben-xvi_enc_20090629_caritas-inveritate_en.html. Take the Plunge! –(…) –Today patent pools are a favoured system in technology sectors that require common standards, such as the MPEG-2, DVDvideo, DVD-ROM and radio. Medicines, though, are trickier terrain. –(…) –UNITAID may be able to pull it off with some luck and lots of hard work. (…) They have a delicate and onerous task before them. Millions of people are waiting hopefully at the patent poolside." –Latha Jishnu/ New Delhi July 23, 2008 What next?


































![UNITAID Prospectus] and it is this text that includes language taking](http://s1.studyres.com/store/data/000725870_1-a6691778ed3e3f5a3571caea06e5de93-150x150.png)

